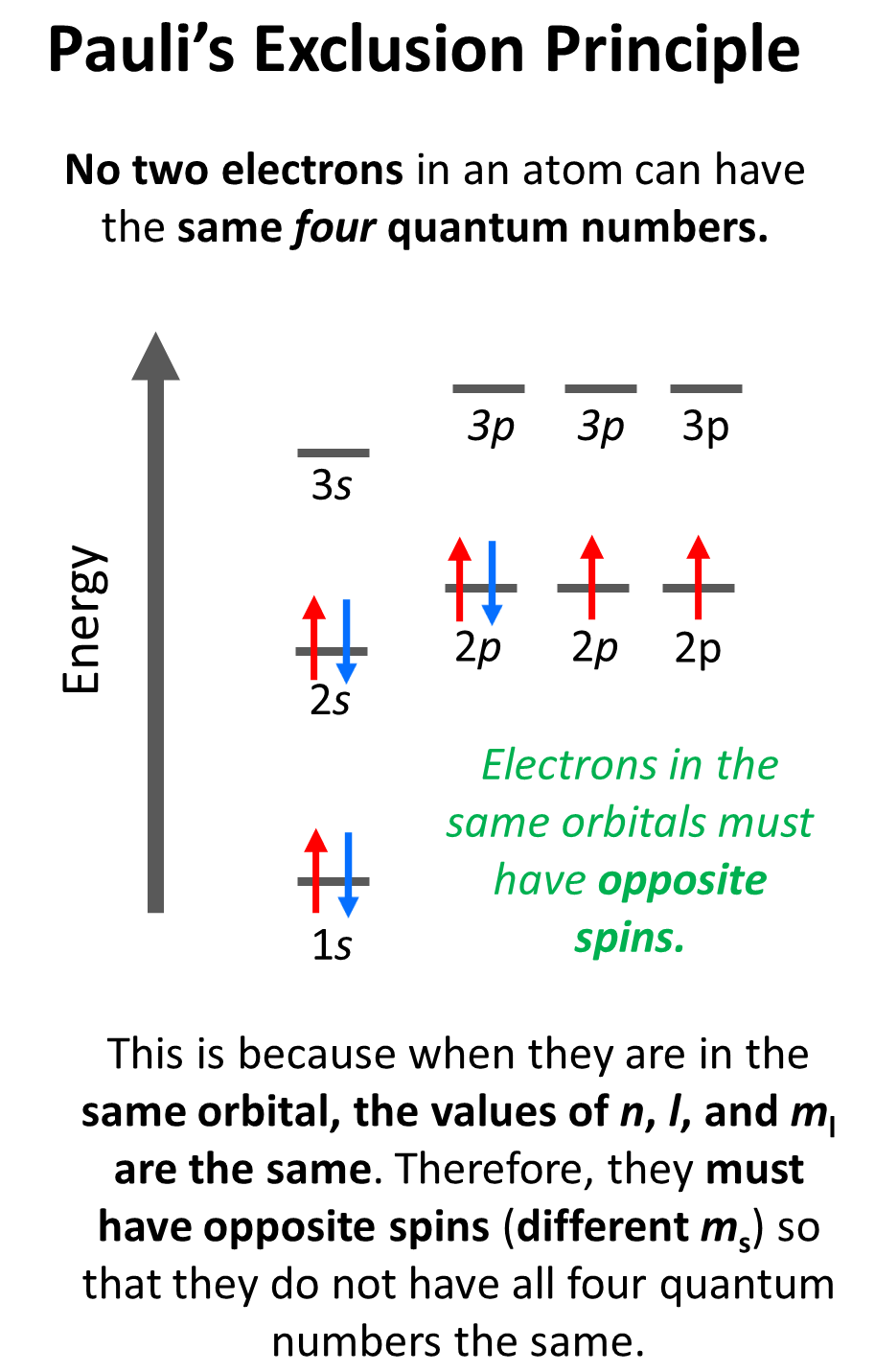
What Is The Difference Between Hund S Rule And Aufbau Principle . If orbitals of equal energy (i.e. Lower energy orbitals fill before higher energy orbitals. Hund's rule of maximum multiplicity. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. One electron goes into each. when assigning electrons to orbitals, we must follow a set of three rules: in simple terms, the aufbau principle means fill the orbitals from bottom to top. hund's rule explained. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill.
from general.chemistrysteps.com
Lower energy orbitals fill before higher energy orbitals. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. Hund's rule of maximum multiplicity. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. hund's rule explained. If orbitals of equal energy (i.e. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. when assigning electrons to orbitals, we must follow a set of three rules: in simple terms, the aufbau principle means fill the orbitals from bottom to top. One electron goes into each.
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle
What Is The Difference Between Hund S Rule And Aufbau Principle the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. Lower energy orbitals fill before higher energy orbitals. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. One electron goes into each. If orbitals of equal energy (i.e. hund's rule explained. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. when assigning electrons to orbitals, we must follow a set of three rules: in simple terms, the aufbau principle means fill the orbitals from bottom to top. Hund's rule of maximum multiplicity.
From www.youtube.com
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle What Is The Difference Between Hund S Rule And Aufbau Principle hund's rule explained. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. in simple terms, the aufbau principle means fill the orbitals from bottom to top. Lower energy orbitals fill before higher energy orbitals. Hund's rule of maximum multiplicity. when assigning electrons. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.tes.com
Pauli Exclusion Principle, Aufbau and Hund's Rule Grade 12 Chemistry What Is The Difference Between Hund S Rule And Aufbau Principle If orbitals of equal energy (i.e. when assigning electrons to orbitals, we must follow a set of three rules: in simple terms, the aufbau principle means fill the orbitals from bottom to top. Lower energy orbitals fill before higher energy orbitals. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go. What Is The Difference Between Hund S Rule And Aufbau Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund S Rule And Aufbau Principle hund's rule explained. Hund's rule of maximum multiplicity. in simple terms, the aufbau principle means fill the orbitals from bottom to top. Lower energy orbitals fill before higher energy orbitals. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. One electron goes into each. If orbitals of equal. What Is The Difference Between Hund S Rule And Aufbau Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund S Rule And Aufbau Principle when assigning electrons to orbitals, we must follow a set of three rules: If orbitals of equal energy (i.e. Hund's rule of maximum multiplicity. hund's rule explained. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. Lower energy orbitals fill before higher energy orbitals. in simple terms, the aufbau principle means. What Is The Difference Between Hund S Rule And Aufbau Principle.
From mungfali.com
Aufbau Principle Hund's Rule What Is The Difference Between Hund S Rule And Aufbau Principle when assigning electrons to orbitals, we must follow a set of three rules: If orbitals of equal energy (i.e. in simple terms, the aufbau principle means fill the orbitals from bottom to top. One electron goes into each. Hund's rule of maximum multiplicity. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling.. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.youtube.com
Aufbau principal Pauli exclusion principle, hund's rule Simplified What Is The Difference Between Hund S Rule And Aufbau Principle Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. One electron goes into each. Hund's rule of maximum multiplicity. If orbitals of equal energy (i.e. Lower energy orbitals fill before higher energy orbitals. hund's. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.chemistrylearner.com
Hund’s Rule Statement, Definition, and Example. What Is The Difference Between Hund S Rule And Aufbau Principle Hund's rule of maximum multiplicity. Lower energy orbitals fill before higher energy orbitals. One electron goes into each. when assigning electrons to orbitals, we must follow a set of three rules: aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. hund's rule explained. in simple terms, the. What Is The Difference Between Hund S Rule And Aufbau Principle.
From mungfali.com
Aufbau Principle Hund's Rule What Is The Difference Between Hund S Rule And Aufbau Principle Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. If orbitals of equal energy (i.e. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. Lower energy orbitals fill before higher energy orbitals. One electron goes into each. hund's. What Is The Difference Between Hund S Rule And Aufbau Principle.
From exytrgfmy.blob.core.windows.net
What Is The Difference Between Hund S Rule And Pauli Exclusion What Is The Difference Between Hund S Rule And Aufbau Principle when assigning electrons to orbitals, we must follow a set of three rules: in simple terms, the aufbau principle means fill the orbitals from bottom to top. One electron goes into each. Hund's rule of maximum multiplicity. hund's rule explained. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go. What Is The Difference Between Hund S Rule And Aufbau Principle.
From enginelibmisallying.z14.web.core.windows.net
What Is The Hund's Rule In Chemistry What Is The Difference Between Hund S Rule And Aufbau Principle Lower energy orbitals fill before higher energy orbitals. when assigning electrons to orbitals, we must follow a set of three rules: the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. If orbitals of equal energy (i.e. Hund's rule of maximum multiplicity. Fill the atomic. What Is The Difference Between Hund S Rule And Aufbau Principle.
From pediaa.com
What is the Difference Between Pauli Exclusion Principle and Hund's What Is The Difference Between Hund S Rule And Aufbau Principle If orbitals of equal energy (i.e. hund's rule explained. in simple terms, the aufbau principle means fill the orbitals from bottom to top. Lower energy orbitals fill before higher energy orbitals. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.youtube.com
2.3 a Aufbau principle, Hund's rule and Pauli exclusion principle YouTube What Is The Difference Between Hund S Rule And Aufbau Principle aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. hund's rule explained. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which. What Is The Difference Between Hund S Rule And Aufbau Principle.
From pediaa.com
Difference Between Aufbau Principle and Hund's Rule Theory What Is The Difference Between Hund S Rule And Aufbau Principle hund's rule explained. Lower energy orbitals fill before higher energy orbitals. when assigning electrons to orbitals, we must follow a set of three rules: If orbitals of equal energy (i.e. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. aufbau’s principle, hund’s. What Is The Difference Between Hund S Rule And Aufbau Principle.
From chemistnotes.com
Aufbau Principle Definition, Example, and Limitations Chemistry Notes What Is The Difference Between Hund S Rule And Aufbau Principle hund's rule explained. Hund's rule of maximum multiplicity. Lower energy orbitals fill before higher energy orbitals. One electron goes into each. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. in simple terms, the aufbau principle means fill the orbitals from bottom to top. the aufbau principle. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.reddit.com
Aufbau principle and hund’s rule r/chemistry What Is The Difference Between Hund S Rule And Aufbau Principle Hund's rule of maximum multiplicity. hund's rule explained. If orbitals of equal energy (i.e. Lower energy orbitals fill before higher energy orbitals. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. when assigning electrons to orbitals, we must follow a set of three. What Is The Difference Between Hund S Rule And Aufbau Principle.
From sciencenotes.org
Hund's Rule Definition and Examples What Is The Difference Between Hund S Rule And Aufbau Principle the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. Lower energy orbitals fill before higher energy orbitals. in simple terms, the aufbau principle means fill the orbitals from bottom to top. hund's rule explained. One electron goes into each. when assigning electrons. What Is The Difference Between Hund S Rule And Aufbau Principle.
From facts.net
18 Intriguing Facts About Hund's Rule What Is The Difference Between Hund S Rule And Aufbau Principle Lower energy orbitals fill before higher energy orbitals. hund's rule explained. One electron goes into each. in simple terms, the aufbau principle means fill the orbitals from bottom to top. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. If orbitals of equal energy (i.e. the aufbau. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.vrogue.co
Difference Between Aufbau Principle And Hund S Rule T vrogue.co What Is The Difference Between Hund S Rule And Aufbau Principle One electron goes into each. in simple terms, the aufbau principle means fill the orbitals from bottom to top. when assigning electrons to orbitals, we must follow a set of three rules: aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. hund's rule explained. the aufbau. What Is The Difference Between Hund S Rule And Aufbau Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund S Rule And Aufbau Principle One electron goes into each. in simple terms, the aufbau principle means fill the orbitals from bottom to top. when assigning electrons to orbitals, we must follow a set of three rules: If orbitals of equal energy (i.e. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order. What Is The Difference Between Hund S Rule And Aufbau Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund S Rule And Aufbau Principle the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. in simple terms, the aufbau principle means fill the orbitals from bottom to top. when assigning electrons to orbitals, we must follow a set of three rules: Lower energy orbitals fill before higher energy. What Is The Difference Between Hund S Rule And Aufbau Principle.
From pediaa.com
Difference Between Aufbau Principle and Hund's Rule Theory What Is The Difference Between Hund S Rule And Aufbau Principle the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. when assigning electrons to orbitals, we must follow a set of three rules: aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. One electron goes. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.youtube.com
Structure of atom class 10 Pauli exclusion principle Aufbau What Is The Difference Between Hund S Rule And Aufbau Principle the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. in simple terms, the aufbau principle means fill the orbitals from bottom to top. when. What Is The Difference Between Hund S Rule And Aufbau Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund S Rule And Aufbau Principle Hund's rule of maximum multiplicity. when assigning electrons to orbitals, we must follow a set of three rules: Lower energy orbitals fill before higher energy orbitals. hund's rule explained. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. Fill the atomic orbitals with. What Is The Difference Between Hund S Rule And Aufbau Principle.
From paininmyassproject.weebly.com
The Aufbau Principle Atomic Theory Timeline What Is The Difference Between Hund S Rule And Aufbau Principle One electron goes into each. Lower energy orbitals fill before higher energy orbitals. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. in simple terms, the aufbau principle means. What Is The Difference Between Hund S Rule And Aufbau Principle.
From exytrgfmy.blob.core.windows.net
What Is The Difference Between Hund S Rule And Pauli Exclusion What Is The Difference Between Hund S Rule And Aufbau Principle the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. when assigning electrons to orbitals, we must follow a set of three rules: Lower energy orbitals fill before higher energy. What Is The Difference Between Hund S Rule And Aufbau Principle.
From ditki.com
Biochemistry Glossary Energy Aufbau Principle & Hund's Rule ditki What Is The Difference Between Hund S Rule And Aufbau Principle If orbitals of equal energy (i.e. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. Hund's rule of maximum multiplicity. Fill the atomic orbitals with electrons. What Is The Difference Between Hund S Rule And Aufbau Principle.
From exytrgfmy.blob.core.windows.net
What Is The Difference Between Hund S Rule And Pauli Exclusion What Is The Difference Between Hund S Rule And Aufbau Principle If orbitals of equal energy (i.e. Hund's rule of maximum multiplicity. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. in simple terms, the aufbau principle means fill the. What Is The Difference Between Hund S Rule And Aufbau Principle.
From askfilo.com
Difference between aufbau principal and hunds rule Filo What Is The Difference Between Hund S Rule And Aufbau Principle Lower energy orbitals fill before higher energy orbitals. hund's rule explained. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. One electron goes into each. If orbitals of equal energy (i.e. when assigning electrons to orbitals, we must follow a set of three rules: Hund's rule of maximum. What Is The Difference Between Hund S Rule And Aufbau Principle.
From scienceinfo.com
Hund's Rule of Maximum Multiplicity Explanation, Importance, Examples What Is The Difference Between Hund S Rule And Aufbau Principle Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. One electron goes into each. when assigning electrons to orbitals, we must follow a set of three rules: If orbitals of equal energy (i.e. Lower energy orbitals fill before higher energy orbitals. the aufbau principle and hund’s rule both describe how electrons fill. What Is The Difference Between Hund S Rule And Aufbau Principle.
From circuitdbfrequents.z19.web.core.windows.net
Aufbau Principle And Hund's Rule What Is The Difference Between Hund S Rule And Aufbau Principle If orbitals of equal energy (i.e. Lower energy orbitals fill before higher energy orbitals. One electron goes into each. Hund's rule of maximum multiplicity. hund's rule explained. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. Fill the atomic orbitals with electrons starting at the lowest available energy states. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.slideserve.com
PPT Hund’s Rule PowerPoint Presentation, free download ID562489 What Is The Difference Between Hund S Rule And Aufbau Principle If orbitals of equal energy (i.e. in simple terms, the aufbau principle means fill the orbitals from bottom to top. Hund's rule of maximum multiplicity. hund's rule explained. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. aufbau’s principle, hund’s rule, and. What Is The Difference Between Hund S Rule And Aufbau Principle.
From chemistnotes.com
Hund's Rule, Definition, Example, and Application Chemistry Notes What Is The Difference Between Hund S Rule And Aufbau Principle If orbitals of equal energy (i.e. the aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill. hund's rule explained. One electron goes into each. in simple terms, the aufbau principle means fill the orbitals from bottom to top. Hund's rule of maximum multiplicity. . What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.youtube.com
Electron Configuration (Aufbau principle and Hund's rule) YouTube What Is The Difference Between Hund S Rule And Aufbau Principle Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. in simple terms, the aufbau principle means fill the orbitals from bottom to top. If orbitals of equal energy (i.e. when assigning electrons to orbitals, we must follow a set of three rules: One electron goes into each. hund's rule explained. Hund's. What Is The Difference Between Hund S Rule And Aufbau Principle.
From app.jove.com
The Aufbau Principle and Hund's Rule Concept Chemistry JoVe What Is The Difference Between Hund S Rule And Aufbau Principle hund's rule explained. If orbitals of equal energy (i.e. aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the. in simple terms, the aufbau principle means fill the orbitals from bottom to top. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. One. What Is The Difference Between Hund S Rule And Aufbau Principle.
From www.youtube.com
The Given Electronic Configuration violates Hund's Rule, Aufbau What Is The Difference Between Hund S Rule And Aufbau Principle hund's rule explained. Fill the atomic orbitals with electrons starting at the lowest available energy states before filling. Hund's rule of maximum multiplicity. One electron goes into each. when assigning electrons to orbitals, we must follow a set of three rules: aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go. What Is The Difference Between Hund S Rule And Aufbau Principle.