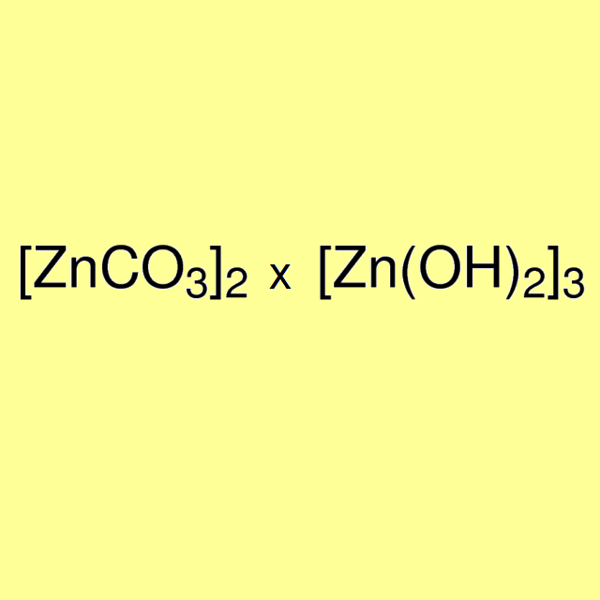Zinc Carbonate With Calcination . the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. It is referred to as smithsonite or calamine or zinc spar. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc.
from www.limac.lv
the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. It is referred to as smithsonite or calamine or zinc spar. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc.
Zn Catalog LiMac Science
Zinc Carbonate With Calcination When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. It is referred to as smithsonite or calamine or zinc spar.
From yurunchemical.en.made-in-china.com
Zinc Carbonate Zinc Carbonate and Animal Feed Additive Zinc Carbonate Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was.. Zinc Carbonate With Calcination.
From www.youtube.com
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube Zinc Carbonate With Calcination In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. When zinc carbonate is heated in the calcination process, it. Zinc Carbonate With Calcination.
From sielc.com
Zinc carbonate SIELC Zinc Carbonate With Calcination the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. It is referred to as smithsonite or calamine or zinc spar. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc. Zinc Carbonate With Calcination.
From www.youtube.com
UK GCSE Chemistry Topic 1 Zinc Carbonate YouTube Zinc Carbonate With Calcination the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. In pure form, it is colourless and transparent. Zinc Carbonate With Calcination.
From brainly.in
Write chemical reaction for each (1) Zinc carbonate is calcinated. (2 Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. When zinc carbonate is heated in the calcination process,. Zinc Carbonate With Calcination.
From brainly.in
Chemical formula of zinc carbonate with solution Brainly.in Zinc Carbonate With Calcination In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc. Zinc Carbonate With Calcination.
From www.youtube.com
How to write chemical formula of zinc carbonateMolecular formula of Zinc Carbonate With Calcination zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. the reaction process of. Zinc Carbonate With Calcination.
From www.youtube.com
How to Write the Formula for ZnCO3 (Zinc carbonate) YouTube Zinc Carbonate With Calcination the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. It is referred to as smithsonite or calamine or zinc spar. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc.. Zinc Carbonate With Calcination.
From www.indiamart.com
ZnCo3 Zinc Carbonate, Grade Standard Indutrial at Rs 115/kg in Rajkot Zinc Carbonate With Calcination in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. When zinc carbonate is heated in the calcination process, it is. Zinc Carbonate With Calcination.
From www.chemone.com
Zinc Carbonate Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. In pure form, it is colourless and transparent but more frequently coloured by the presence of. Zinc Carbonate With Calcination.
From www.indiamart.com
Zinc Carbonate ZnCo3, Grade Standard Indutrial at Rs 148/kg in Rajkot Zinc Carbonate With Calcination the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. When zinc carbonate is heated in the calcination process,. Zinc Carbonate With Calcination.
From www.snapdeal.com
ZINC CARBONATE (basic) (CAS NO.5263025) 500 GM Buy Online at Best Zinc Carbonate With Calcination in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. the. Zinc Carbonate With Calcination.
From www.academia.edu
(PDF) Photoactive and nonhazardous kaolin/ZnO composites prepared by Zinc Carbonate With Calcination zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. When zinc carbonate is heated in the calcination process, it. Zinc Carbonate With Calcination.
From testbook.com
Zinc Carbonate Learn its Formula, Structure, Properties & Uses Zinc Carbonate With Calcination zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. zinc oxide. Zinc Carbonate With Calcination.
From sonuchem.com
Zinc Carbonate Sonu Chemical Zinc Carbonate With Calcination zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. zinc oxide. Zinc Carbonate With Calcination.
From www.researchgate.net
XRD patterns of zinc oxide nanoparticles obtained by calcination of the Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. zinc oxide as a widely used material in various industries was prepared by calcination. Zinc Carbonate With Calcination.
From www.indiamart.com
Zinc Carbonate Basic at best price in Vadodara by Axiom Chemicals Zinc Carbonate With Calcination the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. zinc oxide as a widely. Zinc Carbonate With Calcination.
From fyoupaopr.blob.core.windows.net
Zinc Carbonate Layer at Jasmine Ross blog Zinc Carbonate With Calcination In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. It is referred to. Zinc Carbonate With Calcination.
From www.ddftengineering.com
Active Zinc Oxide Calciner Zinc Carbonate Calcination Equipment Buy Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. zinc oxide as a widely used material. Zinc Carbonate With Calcination.
From www.youtube.com
Synthesis of basic Zinc Carbonate YouTube Zinc Carbonate With Calcination during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. It is referred to as smithsonite or calamine or zinc spar. zinc carbonate is a white crystalline powder that occurs. Zinc Carbonate With Calcination.
From www.fishersci.com
Zinc Carbonate, Basic, Powder, Reagent, 65, Spectrum Fisher Scientific Zinc Carbonate With Calcination When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. It is referred to as smithsonite or calamine or zinc spar. zinc carbonate is a white crystalline powder. Zinc Carbonate With Calcination.
From www.researchgate.net
(A) SEM of the zinc oxide particles obtained by calcining zinc basic Zinc Carbonate With Calcination zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. during the. Zinc Carbonate With Calcination.
From www.fishersci.com
Zinc Carbonate, Basic, Powder, Reagent, 65, Spectrum Chemical Fisher Zinc Carbonate With Calcination zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. It. Zinc Carbonate With Calcination.
From www.youtube.com
Roasting,Calcination & Extraction Of ZincPart 12cbsegrade12 Zinc Carbonate With Calcination the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. It is referred to as smithsonite or calamine or zinc spar. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to. Zinc Carbonate With Calcination.
From www.researchgate.net
Figure S6 TG of assynthesized mixed cobaltzinc carbonate synthesized Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. zinc oxide as a widely used material in various industries was prepared by. Zinc Carbonate With Calcination.
From www.glochem.com
Exploring the Diverse Applications of Zinc Carbonate From Zinc Carbonate With Calcination zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. zinc oxide as. Zinc Carbonate With Calcination.
From www.slideserve.com
PPT Global Zinc Carbonate Basic Market PowerPoint Presentation, free Zinc Carbonate With Calcination the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. It is referred to as smithsonite or calamine or zinc spar. zinc oxide as a widely used material in various industries was prepared by calcination. Zinc Carbonate With Calcination.
From www.researchgate.net
Main product of the calcination of carbonate rocks from the waste piles Zinc Carbonate With Calcination during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. It is referred to as smithsonite or calamine or zinc spar. When zinc carbonate is heated in the calcination process, it is. Zinc Carbonate With Calcination.
From brainly.in
Give chemical equation for the following first. 1 when zinc carbonate Zinc Carbonate With Calcination zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. the reaction process of nanometer zno preparation through basic zinc carbonate calcination was. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted. In pure form, it is colourless and. Zinc Carbonate With Calcination.
From brainly.in
Write chemical reaction for each (1) Zinc carbonate is calcinated. (2 Zinc Carbonate With Calcination in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. It is referred to as smithsonite or. Zinc Carbonate With Calcination.
From glchems.en.made-in-china.com
Industrial Basic Zinc Carbonate Powder / Basic Zinc Carbonate China Zinc Carbonate With Calcination In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. When zinc carbonate is heated in the calcination process, it is converted into zinc oxide and then it is easily converted.. Zinc Carbonate With Calcination.
From www.limac.lv
Zn Catalog LiMac Science Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. In pure form, it is colourless and transparent but more frequently coloured by the presence of iron, manganese, copper, etc. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. in this study the thermal calcination of two materials, high. Zinc Carbonate With Calcination.
From chempedia.info
Zinc carbonate, water reactions Big Chemical Encyclopedia Zinc Carbonate With Calcination It is referred to as smithsonite or calamine or zinc spar. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. the reaction process of nanometer zno preparation through basic zinc. Zinc Carbonate With Calcination.
From vanspa.vn
Zinc Carbonate Zinc Carbonate With Calcination in this study the thermal calcination of two materials, high purity zinc carbonate hydroxide and the calsimin zinc. during the process of calcination, z inc carbonate znco 3 further undergoes decomposition in order to form zinc oxide. It is referred to as smithsonite or calamine or zinc spar. zinc carbonate is a white crystalline powder that occurs. Zinc Carbonate With Calcination.
From nanografi.com
Zinc Carbonate Properties and Applications Nanografi Nano Technology Zinc Carbonate With Calcination zinc carbonate is a white crystalline powder that occurs naturally as granular or earthy masses. zinc oxide as a widely used material in various industries was prepared by calcination of precursor (zinc. It is referred to as smithsonite or calamine or zinc spar. In pure form, it is colourless and transparent but more frequently coloured by the presence. Zinc Carbonate With Calcination.