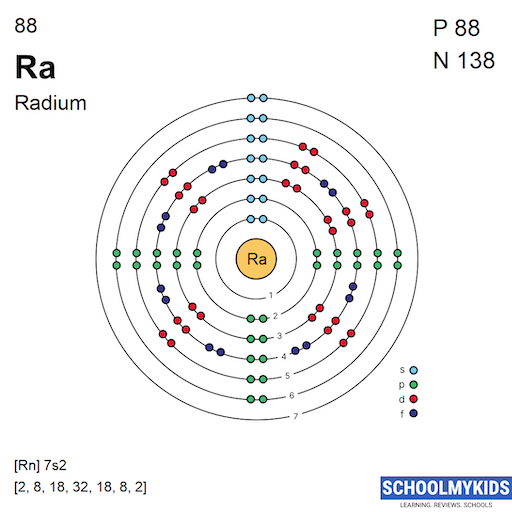
What Are Properties Of Radium . Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium is an alkaline earth metal. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium has 33 isotopes, ranging in mass number 202 to 234. Its physical and chemical properties most closely resemble. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. It exhibits several notable chemical. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Radium is approximately 2.7 million times more radioactive compared to. Pure radium metal is bright white.
from www.schoolmykids.com
Its physical and chemical properties most closely resemble. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium has 33 isotopes, ranging in mass number 202 to 234. Radium is approximately 2.7 million times more radioactive compared to. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. It exhibits several notable chemical. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is an alkaline earth metal.
Radium (Ra) Element Information, Facts, Properties, Uses Periodic
What Are Properties Of Radium It exhibits several notable chemical. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium is approximately 2.7 million times more radioactive compared to. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium has 33 isotopes, ranging in mass number 202 to 234. Radium is an alkaline earth metal. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. It exhibits several notable chemical. Pure radium metal is bright white. Its physical and chemical properties most closely resemble. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2.
From www.slideserve.com
PPT Radium PowerPoint Presentation ID2424142 What Are Properties Of Radium Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Its physical and chemical properties most closely resemble. Radium is approximately 2.7 million times more radioactive compared to. Radium is an alkaline earth metal. Radium has a melting point of 700°c, boiling. What Are Properties Of Radium.
From www.examples.com
Radium (Ra) Definition, Preparation, Properties, Uses, Compounds What Are Properties Of Radium It exhibits several notable chemical. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is approximately 2.7 million times more radioactive compared to. Radium is an alkaline earth metal. Its physical. What Are Properties Of Radium.
From www.shutterstock.com
Radium Properties Electron Configurationvector Illustration Stock What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is an alkaline earth metal. Pure radium metal is bright white. Radium has 33 isotopes, ranging in mass number 202 to 234. Its physical and chemical properties most closely resemble. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. What Are Properties Of Radium.
From www.sliderbase.com
Properties of Radium What Are Properties Of Radium Pure radium metal is bright white. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium has 33 isotopes, ranging in mass number 202 to 234. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. It exhibits several notable chemical. Radium is the heaviest known alkaline earth metal and is. What Are Properties Of Radium.
From www.haikudeck.com
Radium by Leta Lohrmeyer What Are Properties Of Radium Pure radium metal is bright white. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. It exhibits several notable chemical. Radium. What Are Properties Of Radium.
From www.americanelements.com
Radium (Ra) AMERICAN ELEMENTS What Are Properties Of Radium Radium is approximately 2.7 million times more radioactive compared to. Radium is an alkaline earth metal. It exhibits several notable chemical. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Radium glows in the. What Are Properties Of Radium.
From www.examples.com
Radium (Ra) Definition, Preparation, Properties, Uses, Compounds What Are Properties Of Radium Radium is approximately 2.7 million times more radioactive compared to. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium is an alkaline earth metal. Pure radium metal is bright white. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Its physical and chemical properties most closely resemble. It exhibits several notable. What Are Properties Of Radium.
From eduinput.com
RadiumDiscovery, Properties, And Applications What Are Properties Of Radium Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Its physical and chemical properties most closely resemble. It exhibits several notable chemical. Pure radium metal is bright white. Radium is an alkaline earth metal. Radium is a highly reactive and radioactive element,. What Are Properties Of Radium.
From www.chemistrylearner.com
Radium Facts, Symbol, Discovery, Properties, Uses What Are Properties Of Radium Radium is an alkaline earth metal. Its physical and chemical properties most closely resemble. Pure radium metal is bright white. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium has 33. What Are Properties Of Radium.
From www.istockphoto.com
Electron Configuration Of Radium Illustrations, RoyaltyFree Vector What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. It exhibits several notable. What Are Properties Of Radium.
From www.slideserve.com
PPT Radium PowerPoint Presentation, free download ID1924630 What Are Properties Of Radium Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Pure radium metal is bright white. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the. What Are Properties Of Radium.
From stock.adobe.com
Radium Properties and Electron ConfigurationVector illustration Stock What Are Properties Of Radium Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Radium has 33 isotopes, ranging in mass number 202 to 234. Pure radium metal is bright white. Its physical and chemical properties most closely resemble. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium has a melting point of 700°c,. What Are Properties Of Radium.
From www.schoolmykids.com
Radium (Ra) Element Information, Facts, Properties, Uses Periodic What Are Properties Of Radium Radium is approximately 2.7 million times more radioactive compared to. It exhibits several notable chemical. Radium is an alkaline earth metal. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium has 33 isotopes, ranging in mass number 202 to 234. Pure radium metal is bright white. Radium is a highly reactive and radioactive element, categorized under the alkaline. What Are Properties Of Radium.
From www.schoolmykids.com
Radium (Ra) Element Information, Facts, Properties, Uses Periodic What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Pure radium metal is bright white. Its physical and chemical properties most closely resemble. Radium is an alkaline earth metal. It exhibits several. What Are Properties Of Radium.
From matmake.com
Radium (Ra) Properties What Are Properties Of Radium Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Pure radium metal is bright white. It exhibits several notable chemical. Radium has 33 isotopes, ranging in mass number 202 to 234. Radium is an alkaline earth metal. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium has a melting point. What Are Properties Of Radium.
From exopsskcd.blob.core.windows.net
Key Properties Of Radium at Charles Stroud blog What Are Properties Of Radium Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Pure radium metal is bright white. Radium is a highly reactive and radioactive element, categorized under the. What Are Properties Of Radium.
From www.sliderbase.com
Properties of Radium What Are Properties Of Radium Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. It exhibits several notable chemical. Radium is approximately 2.7 million times more radioactive compared to. Sources, facts, uses, scarcity (sri), podcasts, alchemical. What Are Properties Of Radium.
From www.istockphoto.com
Ra Radium Element Information Facts Properties Trends Uses And What Are Properties Of Radium Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium has 33 isotopes, ranging in mass number 202 to 234. Radium is an alkaline earth metal. Radium is approximately 2.7 million times more radioactive compared. What Are Properties Of Radium.
From www.thoughtco.com
Radium Facts and Chemical and Physical Properties What Are Properties Of Radium Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Pure radium metal is bright white. Radium is approximately 2.7 million times more radioactive compared to. Its physical and chemical properties most closely resemble. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of. What Are Properties Of Radium.
From mintteam861.weebly.com
Radium Atomic Number mintteam What Are Properties Of Radium Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Its physical and chemical properties most closely resemble. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Pure radium metal is bright white. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium is approximately. What Are Properties Of Radium.
From www.examples.com
Radium (Ra) Definition, Preparation, Properties, Uses, Compounds What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Its physical and chemical properties most closely resemble. Radium is approximately 2.7 million times more radioactive compared to. Radium has 33. What Are Properties Of Radium.
From www.scribd.com
The Deadly yet Beneficial Properties of Radium A Presentation on the What Are Properties Of Radium Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Pure radium metal is bright white. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Its physical and chemical properties most closely resemble. Radium is the heaviest known alkaline earth metal and is the only. What Are Properties Of Radium.
From www.nuclear-power.com
What is Radium Properties of Radium Element Symbol Ra nuclear What Are Properties Of Radium Radium is approximately 2.7 million times more radioactive compared to. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Pure radium metal is bright white. It exhibits several notable chemical. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2.. What Are Properties Of Radium.
From www.pinterest.com
Kids learn about the element radium and its chemistry including atomic What Are Properties Of Radium Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium is an alkaline earth metal. Pure radium metal is bright white. Radium has 33 isotopes, ranging in mass number 202 to 234. It exhibits several notable chemical. Radium has a melting point of. What Are Properties Of Radium.
From www.sciencephoto.com
Radium, atomic structure Stock Image C018/3769 Science Photo Library What Are Properties Of Radium It exhibits several notable chemical. Pure radium metal is bright white. Its physical and chemical properties most closely resemble. Radium is approximately 2.7 million times more radioactive compared to. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is the heaviest known alkaline earth metal and is. What Are Properties Of Radium.
From material-properties.org
Radium Periodic Table and Atomic Properties What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Pure radium metal is bright white. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Radium glows in the dark due to its intense radioactivity, which it imparts to other. What Are Properties Of Radium.
From scienceinfo.com
Radium (Ra) Element Properties, Reaction, Uses, Hazards What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Pure radium metal is. What Are Properties Of Radium.
From www.chemistrylearner.com
Radium Facts, Symbol, Discovery, Properties, Uses What Are Properties Of Radium Pure radium metal is bright white. Radium is an alkaline earth metal. Radium is approximately 2.7 million times more radioactive compared to. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Its physical and chemical properties most closely resemble. It exhibits several notable chemical. Radium glows in the. What Are Properties Of Radium.
From www.sliderbase.com
Properties of Radium What Are Properties Of Radium Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium has 33 isotopes, ranging in mass number 202 to 234. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium is approximately 2.7 million times more radioactive compared to. Its physical and chemical properties most closely resemble. Radium is an alkaline. What Are Properties Of Radium.
From www.slideserve.com
PPT Radium PowerPoint Presentation, free download ID1924630 What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium has 33 isotopes, ranging in mass number 202 to 234. Radium is an alkaline earth metal. Its physical and chemical properties. What Are Properties Of Radium.
From periodictableguide.com
Radium (Ra) Periodic Table (Element Information & More) What Are Properties Of Radium Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. It exhibits several notable chemical. Radium is an alkaline earth metal. Radium is approximately 2.7 million times more radioactive compared to. Radium glows in the. What Are Properties Of Radium.
From www.vecteezy.com
Radium symbol. Chemical element of the periodic table. Vector What Are Properties Of Radium Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. It exhibits several notable chemical. Pure radium metal is bright white. Radium has 33 isotopes, ranging in mass number 202 to 234. Sources, facts, uses, scarcity (sri),. What Are Properties Of Radium.
From www.vectorstock.com
Diagram representation of the element radium Vector Image What Are Properties Of Radium It exhibits several notable chemical. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Its physical and chemical properties most closely resemble. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances.. What Are Properties Of Radium.
From material-properties.org
Radium Periodic Table and Atomic Properties What Are Properties Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is approximately 2.7 million times more radioactive compared to. Pure radium metal is bright white. Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Its physical and chemical properties. What Are Properties Of Radium.
From www.sliderbase.com
Properties of Radium What Are Properties Of Radium Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Pure radium metal is bright white. Radium glows in the dark due to its intense radioactivity, which it imparts to other. What Are Properties Of Radium.