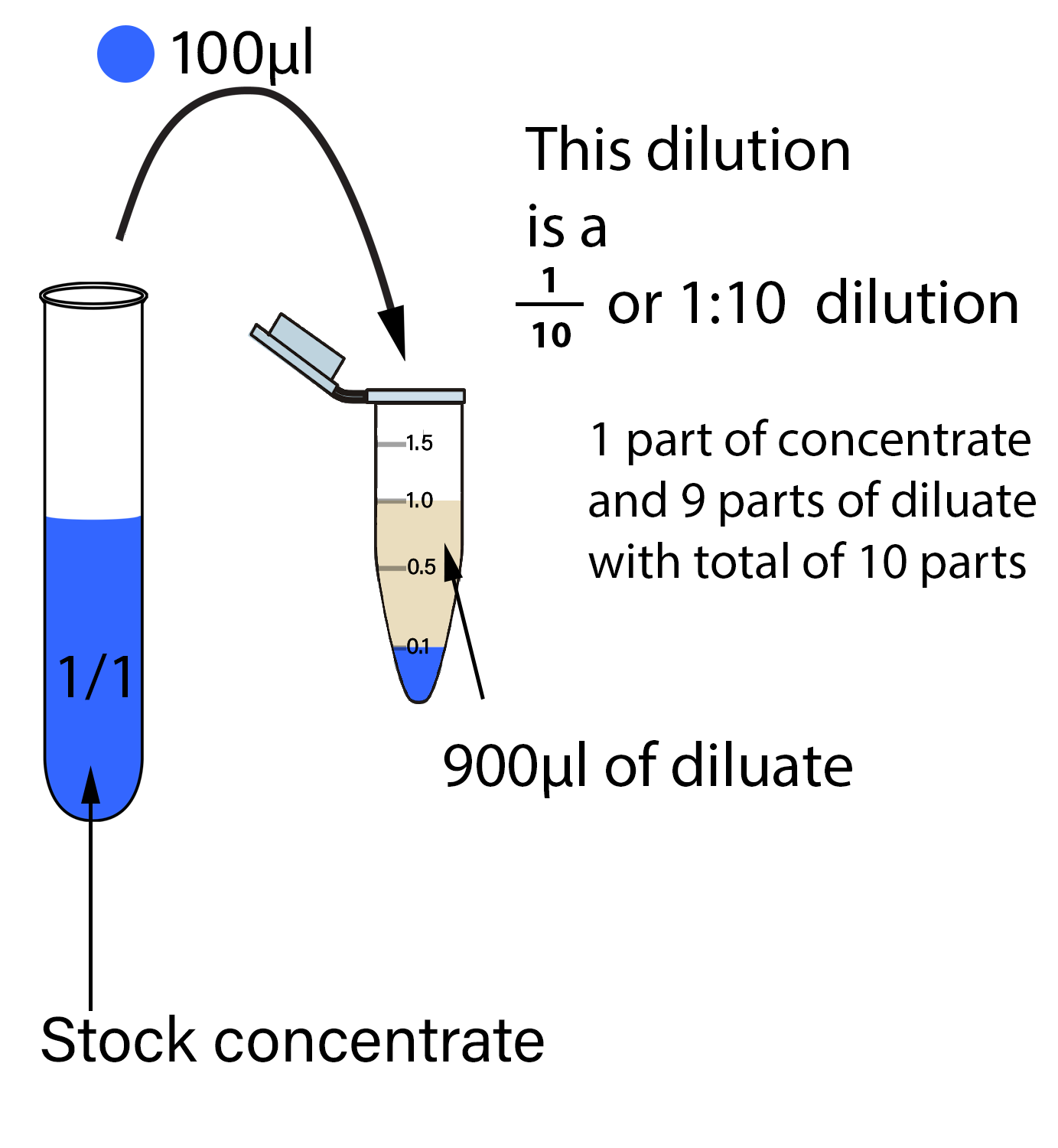
Dilution Calculation Questions . dilution problems, chemistry, molarity & concentration examples, formula & equations changes in concentrations (specifically a reduction) are called dilutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. There’s a bottle of 0.750 m nacl on a shelf. (a) 2.00 l of 18.5 m h 2 so 4,. 3) use the dilution formula: Concentration can be calculated using the. How much of it do you need to prepare 50. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. You should dilute the 133 ml of. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution m 1v 1=m 2v 2 1. calculate the number of moles and the mass of the solute in each of the following solutions: Calculating concentration of a solution.
from exovqdfjo.blob.core.windows.net
calculate the number of moles and the mass of the solute in each of the following solutions: dilution problems, chemistry, molarity & concentration examples, formula & equations You should dilute the 133 ml of. changes in concentrations (specifically a reduction) are called dilutions. Calculating concentration of a solution. Dilution m 1v 1=m 2v 2 1. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. How much of it do you need to prepare 50. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute.
How To Do A Reverse Dilution Calculation at Nathan Cullen blog
Dilution Calculation Questions 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution problems, chemistry, molarity & concentration examples, formula & equations (a) 2.00 l of 18.5 m h 2 so 4,. state whether the concentration of a solution is directly or indirectly proportional to its volume. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. calculate the number of moles and the mass of the solute in each of the following solutions: How much of it do you need to prepare 50. There’s a bottle of 0.750 m nacl on a shelf. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration can be calculated using the. changes in concentrations (specifically a reduction) are called dilutions. 3) use the dilution formula: Dilution m 1v 1=m 2v 2 1. Calculating concentration of a solution. You should dilute the 133 ml of.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Dilution Calculation Questions Concentration can be calculated using the. How much of it do you need to prepare 50. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. changes in concentrations (specifically a reduction) are called dilutions. Dilution m 1v 1=m 2v 2 1. state whether the concentration of a solution is. Dilution Calculation Questions.
From exyzklqnz.blob.core.windows.net
Dilution Calculation Equations at Rebecca Mathis blog Dilution Calculation Questions How much of it do you need to prepare 50. Dilution m 1v 1=m 2v 2 1. changes in concentrations (specifically a reduction) are called dilutions. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. There’s a bottle of 0.750 m nacl on a shelf.. Dilution Calculation Questions.
From exovqdfjo.blob.core.windows.net
How To Do A Reverse Dilution Calculation at Nathan Cullen blog Dilution Calculation Questions dilution problems, chemistry, molarity & concentration examples, formula & equations How much of it do you need to prepare 50. There’s a bottle of 0.750 m nacl on a shelf. state whether the concentration of a solution is directly or indirectly proportional to its volume. (a) 2.00 l of 18.5 m h 2 so 4,. Concentration can be. Dilution Calculation Questions.
From exyzklqnz.blob.core.windows.net
Dilution Calculation Equations at Rebecca Mathis blog Dilution Calculation Questions calculate the number of moles and the mass of the solute in each of the following solutions: 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 3) use the dilution formula: (a) 2.00 l of 18.5 m h 2 so 4,. You should dilute the 133 ml of. Calculating. Dilution Calculation Questions.
From giovqwrja.blob.core.windows.net
Serial Dilution In Microbiology Calculation Method & Technique at Dilution Calculation Questions dilution problems, chemistry, molarity & concentration examples, formula & equations There’s a bottle of 0.750 m nacl on a shelf. Calculating concentration of a solution. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. state whether the concentration of a solution is directly or. Dilution Calculation Questions.
From fyoamjyfe.blob.core.windows.net
What Is Dilution In Chemistry Class 10 at Irene Ochoa blog Dilution Calculation Questions Concentration can be calculated using the. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. Calculating concentration of a solution. a dilution is a process where the concentration of a solution is lowered. Dilution Calculation Questions.
From exyxdeytx.blob.core.windows.net
Dilution Absorbance Calculation at Clyde Rodrigues blog Dilution Calculation Questions dilution problems, chemistry, molarity & concentration examples, formula & equations changes in concentrations (specifically a reduction) are called dilutions. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. 3) use the dilution formula: You should dilute the 133. Dilution Calculation Questions.
From loetrfmxl.blob.core.windows.net
Dilution Equation Question at Gaynell Sampson blog Dilution Calculation Questions Dilution m 1v 1=m 2v 2 1. Calculating concentration of a solution. How much of it do you need to prepare 50. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 3) use the dilution formula: a dilution is a process where the concentration of a solution is lowered. Dilution Calculation Questions.
From exywaipho.blob.core.windows.net
Solution Dilution Calculator Molarity at Laura blog Dilution Calculation Questions Dilution m 1v 1=m 2v 2 1. state whether the concentration of a solution is directly or indirectly proportional to its volume. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. There’s a bottle of 0.750 m nacl on a shelf. Concentration can be calculated. Dilution Calculation Questions.
From www.youtube.com
AS Biology How to calculate serial and simple dilutions YouTube Dilution Calculation Questions There’s a bottle of 0.750 m nacl on a shelf. (a) 2.00 l of 18.5 m h 2 so 4,. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution problems, chemistry, molarity & concentration examples, formula & equations 1) if i have 340 ml of a 0.5 m nabr solution, what. Dilution Calculation Questions.
From www.chegg.com
Solved Name One use of serial dilutions is to calculate the Dilution Calculation Questions 3) use the dilution formula: dilution problems, chemistry, molarity & concentration examples, formula & equations 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. state whether the concentration of a solution is directly or indirectly proportional to its volume. How much of it do you need to prepare. Dilution Calculation Questions.
From studyele1metw.z21.web.core.windows.net
The Mole Concept Worksheets Dilution Calculation Questions 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. calculate the number of moles and the mass of the solute in each of the following solutions: dilution problems, chemistry, molarity & concentration examples, formula & equations Dilution m 1v 1=m 2v 2 1. (a) 2.00 l of 18.5 m. Dilution Calculation Questions.
From www.youtube.com
Microbiology Serial Dilution calculation YouTube Dilution Calculation Questions 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of it do you need to prepare 50. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. 3) use the dilution formula: Calculating concentration of. Dilution Calculation Questions.
From exovqdfjo.blob.core.windows.net
How To Do A Reverse Dilution Calculation at Nathan Cullen blog Dilution Calculation Questions state whether the concentration of a solution is directly or indirectly proportional to its volume. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration can. Dilution Calculation Questions.
From mfawriting332.web.fc2.com
How do you calculate dilution? Dilution Calculation Questions 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. changes in concentrations (specifically a reduction) are called dilutions. M 1 v 1 = m 2 v. Dilution Calculation Questions.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Calculation Questions Calculating concentration of a solution. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. calculate the number of moles and the mass of the solute in each of the following solutions: There’s a bottle of 0.750 m nacl on a shelf. dilution problems,. Dilution Calculation Questions.
From giobnttfq.blob.core.windows.net
Concentration Dilution Calculator Ng/Ul at Kyle McCane blog Dilution Calculation Questions a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. calculate the number of moles and the mass of the solute in each of the following solutions: How much of it do you need to prepare 50. 1) if i have 340 ml of a. Dilution Calculation Questions.
From australianenergy.web.fc2.com
Serial Dilution Lab Conclusion Dilution Calculation Questions Dilution m 1v 1=m 2v 2 1. You should dilute the 133 ml of. Calculating concentration of a solution. Concentration can be calculated using the. There’s a bottle of 0.750 m nacl on a shelf. calculate the number of moles and the mass of the solute in each of the following solutions: How much of it do you need. Dilution Calculation Questions.
From exytogxga.blob.core.windows.net
Dilution Formula Problems at Gary Huston blog Dilution Calculation Questions Dilution m 1v 1=m 2v 2 1. state whether the concentration of a solution is directly or indirectly proportional to its volume. changes in concentrations (specifically a reduction) are called dilutions. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of. Dilution Calculation Questions.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Calculation Questions There’s a bottle of 0.750 m nacl on a shelf. (a) 2.00 l of 18.5 m h 2 so 4,. Concentration can be calculated using the. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. calculate the number of moles and the mass of the solute in each of the. Dilution Calculation Questions.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube Dilution Calculation Questions 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution problems, chemistry, molarity & concentration examples, formula & equations changes in concentrations (specifically a reduction) are called dilutions. Dilution m 1v 1=m 2v 2 1. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) =. Dilution Calculation Questions.
From www.labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Calculation Questions 3) use the dilution formula: calculate the number of moles and the mass of the solute in each of the following solutions: dilution problems, chemistry, molarity & concentration examples, formula & equations Dilution m 1v 1=m 2v 2 1. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if.. Dilution Calculation Questions.
From www.chegg.com
How to solve Pre Laboratory AssignmentAfter reading Dilution Calculation Questions dilution problems, chemistry, molarity & concentration examples, formula & equations How much of it do you need to prepare 50. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Concentration can be calculated using the. There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v. Dilution Calculation Questions.
From giovillwh.blob.core.windows.net
Dilution Factor Cell Counting at Richard Lockett blog Dilution Calculation Questions Dilution m 1v 1=m 2v 2 1. state whether the concentration of a solution is directly or indirectly proportional to its volume. (a) 2.00 l of 18.5 m h 2 so 4,. Calculating concentration of a solution. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620. Dilution Calculation Questions.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Calculation Questions dilution problems, chemistry, molarity & concentration examples, formula & equations (a) 2.00 l of 18.5 m h 2 so 4,. Concentration can be calculated using the. There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. changes in concentrations (specifically a reduction) are called dilutions. 1) if i have 340 ml. Dilution Calculation Questions.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Calculation Questions M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. Concentration can be calculated using the. changes in concentrations (specifically a reduction) are called dilutions. Calculating concentration of a solution. There’s a bottle of 0.750 m nacl on a shelf. (a) 2.00 l of 18.5 m. Dilution Calculation Questions.
From materialmagicpayne.z21.web.core.windows.net
How To Calculate Dilution Concentrations Dilution Calculation Questions dilution problems, chemistry, molarity & concentration examples, formula & equations M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v 2 = 1620 ml. Calculating concentration of a solution. 3) use the dilution formula: a dilution is a process where the concentration of a solution is lowered by. Dilution Calculation Questions.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution Calculation Questions 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution m 1v 1=m 2v 2 1. dilution problems, chemistry, molarity & concentration examples, formula & equations Calculating concentration of a solution. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m) (v 2) v. Dilution Calculation Questions.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Calculation Questions Concentration can be calculated using the. Dilution m 1v 1=m 2v 2 1. dilution problems, chemistry, molarity & concentration examples, formula & equations (a) 2.00 l of 18.5 m h 2 so 4,. changes in concentrations (specifically a reduction) are called dilutions. M 1 v 1 = m 2 v 2 (7.90 m) (133 ml) = (0.648 m). Dilution Calculation Questions.
From fyoezjbli.blob.core.windows.net
Dilution Technique For Blood Volume at Anne Cox blog Dilution Calculation Questions Dilution m 1v 1=m 2v 2 1. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. You should dilute the 133 ml of. 3) use the dilution formula: state whether the concentration of a solution is directly or indirectly proportional to its volume.. Dilution Calculation Questions.
From www.chegg.com
Solved This pour plate of Staphylococcus is after a 106 Dilution Calculation Questions 3) use the dilution formula: changes in concentrations (specifically a reduction) are called dilutions. There’s a bottle of 0.750 m nacl on a shelf. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of it do you need to prepare 50.. Dilution Calculation Questions.
From www.chegg.com
Solved For the following serial dilution fill in the blanks, Dilution Calculation Questions 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 3) use the dilution formula: How much of it do you need to prepare 50. calculate the number of moles and the mass of the solute in each of the following solutions: Dilution m 1v 1=m 2v 2 1. M. Dilution Calculation Questions.