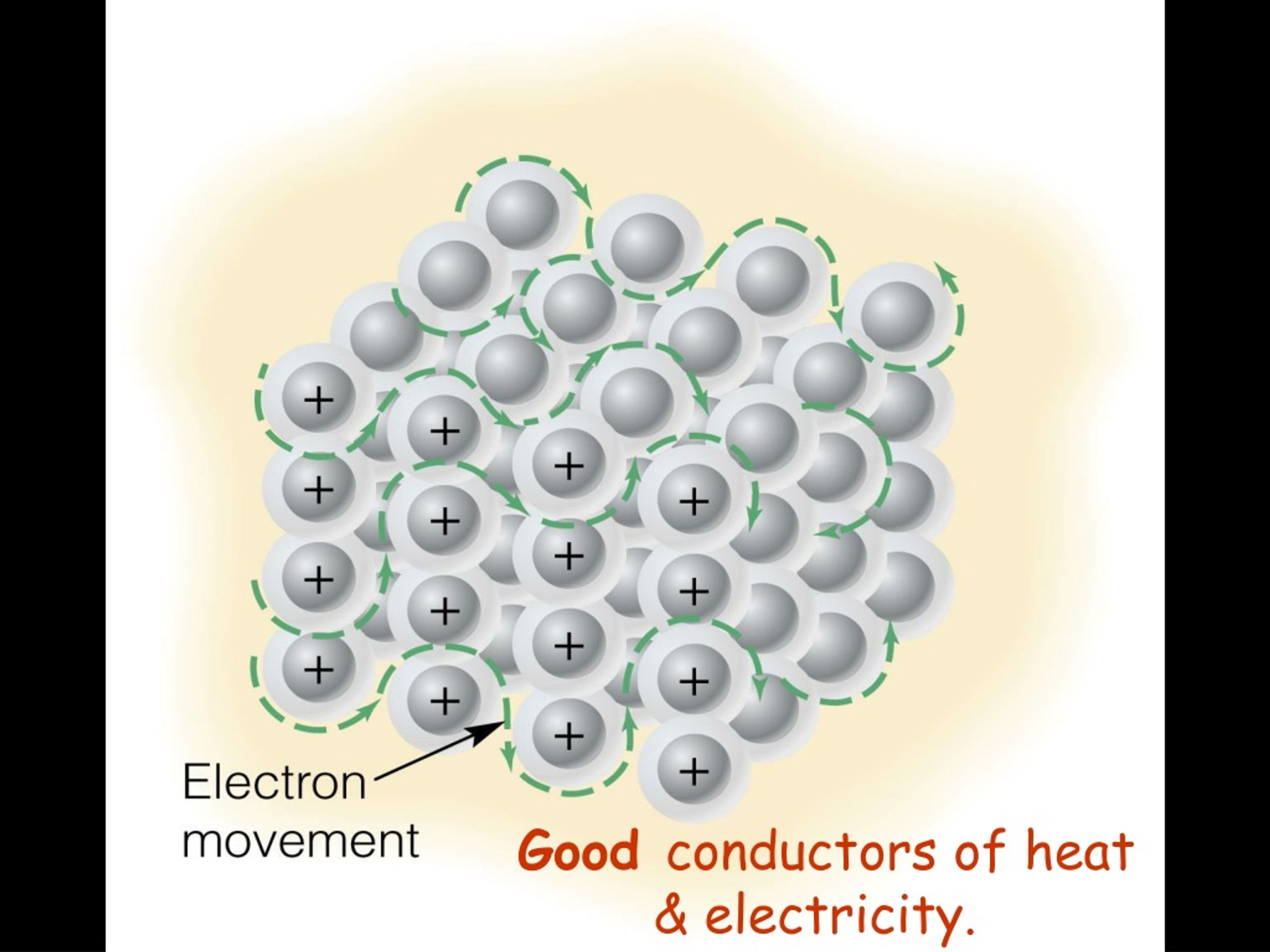
Ionic Is A Good Conductor Of Electricity . Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Substances whose solutions conduct electricity are called electrolytes. Ionic compounds conduct an electric current when melted or dissolved in water. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. They can conduct heat because the kinetic energy itself is the heat carrier. Conductors in their solution form are called ionic conductors. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. For example, saltwater is an ionic solution and is a good. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. All soluble ionic compounds are strong electrolytes.
from www.slideserve.com
Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Substances whose solutions conduct electricity are called electrolytes. All soluble ionic compounds are strong electrolytes. For example, saltwater is an ionic solution and is a good. They can conduct heat because the kinetic energy itself is the heat carrier. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative.
PPT Chapter 7 Properties of Ionic Covalent and Metal Materials
Ionic Is A Good Conductor Of Electricity Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. For example, saltwater is an ionic solution and is a good. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. They can conduct heat because the kinetic energy itself is the heat carrier. All soluble ionic compounds are strong electrolytes. Substances whose solutions conduct electricity are called electrolytes. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Ionic compounds conduct an electric current when melted or dissolved in water. Conductors in their solution form are called ionic conductors. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution.
From eepower.com
Understanding the Electrical Properties of Conductors, Insulators, and Ionic Is A Good Conductor Of Electricity Conductors in their solution form are called ionic conductors. For example, saltwater is an ionic solution and is a good. All soluble ionic compounds are strong electrolytes. Ionic compounds conduct an electric current when melted or dissolved in water. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Ionic compounds are. Ionic Is A Good Conductor Of Electricity.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. For example, saltwater is an ionic solution and is a good. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution.. Ionic Is A Good Conductor Of Electricity.
From slideplayer.com
Ionic/Covalent Bonding Notes ppt download Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. They can conduct heat because the kinetic energy itself is the heat carrier. For example, saltwater is an ionic solution and is a good. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. All soluble ionic. Ionic Is A Good Conductor Of Electricity.
From www.slideserve.com
PPT Chapter 7 Properties of Ionic Covalent and Metal Materials Ionic Is A Good Conductor Of Electricity Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In summary, ionic compounds don't conduct electricity very well because the charge carriers. Ionic Is A Good Conductor Of Electricity.
From www.slideserve.com
PPT Ionic Conductivity and Solid Electrolytes I The Basics Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. For example, saltwater is an ionic solution. Ionic Is A Good Conductor Of Electricity.
From telgurus.co.uk
Why are metals good conductors of electricity? Science Questionnaire Ionic Is A Good Conductor Of Electricity In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. All soluble ionic compounds are strong electrolytes. Ionic compounds conduct an electric current when melted or dissolved in water. In an ionic solution, the \(\ce{a^+}\) ions. Ionic Is A Good Conductor Of Electricity.
From www.slideserve.com
PPT Ionic Conductivity and Solid Electrolytes I The Basics Ionic Is A Good Conductor Of Electricity All soluble ionic compounds are strong electrolytes. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Ionic compounds conduct an electric current when melted or dissolved in water. They can conduct heat because the kinetic energy itself is the heat carrier. Once dissolved or melted, ionic compounds are excellent conductors of electricity and. Ionic Is A Good Conductor Of Electricity.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Conductors in their solution form are called. Ionic Is A Good Conductor Of Electricity.
From slidetodoc.com
Unit 2 Bonding Overview Covalent Bonding Ionic and Ionic Is A Good Conductor Of Electricity In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Substances whose solutions conduct electricity are called electrolytes. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. For example, saltwater is an ionic. Ionic Is A Good Conductor Of Electricity.
From www.researchgate.net
Binaryion conductor and singleion conductor. Schematic illustration Ionic Is A Good Conductor Of Electricity Conductors in their solution form are called ionic conductors. Substances whose solutions conduct electricity are called electrolytes. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. They can conduct heat because the kinetic energy itself is. Ionic Is A Good Conductor Of Electricity.
From www.thoughtco.com
Electrical Conductivity of Metals Ionic Is A Good Conductor Of Electricity In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Conductors in their solution form are called ionic conductors. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and. Ionic Is A Good Conductor Of Electricity.
From joiruwium.blob.core.windows.net
Copper Is A Good Conductor Of Electricity And Heat Justify The Ionic Is A Good Conductor Of Electricity In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Substances whose solutions conduct electricity are called electrolytes. Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. For example, saltwater is an ionic solution and is. Ionic Is A Good Conductor Of Electricity.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the. Ionic Is A Good Conductor Of Electricity.
From slidetodoc.com
Bonding INTRAMOLECULAR FORCES II INTERMOLECULAR FORCES I Chemical Ionic Is A Good Conductor Of Electricity Substances whose solutions conduct electricity are called electrolytes. They can conduct heat because the kinetic energy itself is the heat carrier. For example, saltwater is an ionic solution and is a good. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Strong acids and salts are strong electrolytes because. Ionic Is A Good Conductor Of Electricity.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Ionic Is A Good Conductor Of Electricity They can conduct heat because the kinetic energy itself is the heat carrier. All soluble ionic compounds are strong electrolytes. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ionic compounds conduct an electric current when. Ionic Is A Good Conductor Of Electricity.
From www.science.org
Rectifying ionic current with ionoelastomers Science Ionic Is A Good Conductor Of Electricity In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Substances whose solutions conduct electricity are called electrolytes. Conductors in their solution form are called ionic conductors. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. For. Ionic Is A Good Conductor Of Electricity.
From sciencenotes.org
What Is the Most Conductive Element? Ionic Is A Good Conductor Of Electricity Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Substances whose solutions conduct electricity are called electrolytes. All soluble ionic compounds are strong electrolytes. Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. Ionic Is A Good Conductor Of Electricity.
From slideplayer.com
Mr. Conkey Physical Science Ch ppt download Ionic Is A Good Conductor Of Electricity Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't. Ionic Is A Good Conductor Of Electricity.
From www.pinterest.com
Metal definition An element that is a good conductor of heat and Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Conductors in their solution form are called ionic conductors. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Ionic compounds conduct an electric current when melted or dissolved in water. In summary, ionic compounds don't conduct electricity very well because the charge carriers. Ionic Is A Good Conductor Of Electricity.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Ionic Is A Good Conductor Of Electricity Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Ionic compounds are conductors of electricity when molten or in solution and insulators. Ionic Is A Good Conductor Of Electricity.
From slideplayer.com
Interactions of Matter ppt download Ionic Is A Good Conductor Of Electricity In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Conductors in their solution form are called ionic conductors. Ionic compounds conduct an electric current when melted or dissolved in water. For. Ionic Is A Good Conductor Of Electricity.
From www.youtube.com
Chemical Bonding (Electrical Conductivity of Ionic Compound) Concept Ionic Is A Good Conductor Of Electricity They can conduct heat because the kinetic energy itself is the heat carrier. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Substances whose solutions conduct electricity are called electrolytes. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. All soluble ionic compounds are strong electrolytes. Strong acids and salts are strong. Ionic Is A Good Conductor Of Electricity.
From www.thoughtco.com
10 Examples of Electrical Conductors and Insulators Ionic Is A Good Conductor Of Electricity All soluble ionic compounds are strong electrolytes. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. They can conduct heat because the kinetic energy itself is the heat carrier. Substances whose solutions conduct electricity are called electrolytes. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Conductors. Ionic Is A Good Conductor Of Electricity.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Substances whose solutions conduct electricity are called electrolytes. In. Ionic Is A Good Conductor Of Electricity.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity Ionic Is A Good Conductor Of Electricity Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. For example, saltwater is an ionic solution and is a good. All soluble ionic compounds are strong electrolytes. Ionic compounds conduct an electric current when melted or dissolved in water. Substances whose solutions conduct electricity are called electrolytes. In an ionic solution, the \(\ce{a^+}\). Ionic Is A Good Conductor Of Electricity.
From www.slideserve.com
PPT Chapter 4 Chemical Bonding PowerPoint Presentation, free Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. All soluble ionic compounds are strong electrolytes. They can conduct heat because the kinetic energy itself is the heat carrier. Substances whose solutions conduct electricity are called electrolytes. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the. Ionic Is A Good Conductor Of Electricity.
From slideplayer.com
Ionic Conductivity and Solid Electrolytes I The Basics ppt video Ionic Is A Good Conductor Of Electricity In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Conductors in their solution form are called ionic conductors. Substances whose solutions conduct electricity are called electrolytes. For example, saltwater is an ionic solution and is a good. Ionic compounds are. Ionic Is A Good Conductor Of Electricity.
From www.slideserve.com
PPT Electrical Electrical Conductors & Insulators PowerPoint Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Conductors in their solution form are called ionic conductors. For example, saltwater is an ionic solution and is a good. All soluble ionic compounds are strong electrolytes. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. In an. Ionic Is A Good Conductor Of Electricity.
From brainly.in
Write an activitu to show that ionic compounds are good conductors of Ionic Is A Good Conductor Of Electricity They can conduct heat because the kinetic energy itself is the heat carrier. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds conduct an electric current when melted or dissolved in water. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. In summary, ionic compounds. Ionic Is A Good Conductor Of Electricity.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Ionic Is A Good Conductor Of Electricity They can conduct heat because the kinetic energy itself is the heat carrier. Substances whose solutions conduct electricity are called electrolytes. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Strong. Ionic Is A Good Conductor Of Electricity.
From ec.bertabulle.com
How Do Ions Conduct Electricity Ionic Is A Good Conductor Of Electricity For example, saltwater is an ionic solution and is a good. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. They can conduct heat because the kinetic energy itself is the heat carrier. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. Ionic compounds. Ionic Is A Good Conductor Of Electricity.
From slideplayer.com
SOLUBILITY. ppt download Ionic Is A Good Conductor Of Electricity All soluble ionic compounds are strong electrolytes. Ionic compounds conduct an electric current when melted or dissolved in water. In summary, ionic compounds don't conduct electricity very well because the charge carriers can't move through the crystal. In an ionic solution, the \(\ce{a^+}\) ions migrate toward the negative. Strong acids and salts are strong electrolytes because they completely ionize (dissociate. Ionic Is A Good Conductor Of Electricity.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Ionic Is A Good Conductor Of Electricity Ionic compounds conduct an electric current when melted or dissolved in water. For example, saltwater is an ionic solution and is a good. Substances whose solutions conduct electricity are called electrolytes. All soluble ionic compounds are strong electrolytes. Conductors in their solution form are called ionic conductors. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or. Ionic Is A Good Conductor Of Electricity.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Ionic Is A Good Conductor Of Electricity For example, saltwater is an ionic solution and is a good. All soluble ionic compounds are strong electrolytes. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. They can conduct heat because the kinetic energy itself is the heat carrier. Ionic compounds conduct an electric current when melted or. Ionic Is A Good Conductor Of Electricity.
From slideplayer.com
Chemical Bonding. ppt download Ionic Is A Good Conductor Of Electricity Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Conductors in their solution form are called ionic conductors. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Ionic compounds conduct an electric current when melted or dissolved in water. Substances whose solutions conduct electricity are called electrolytes.. Ionic Is A Good Conductor Of Electricity.