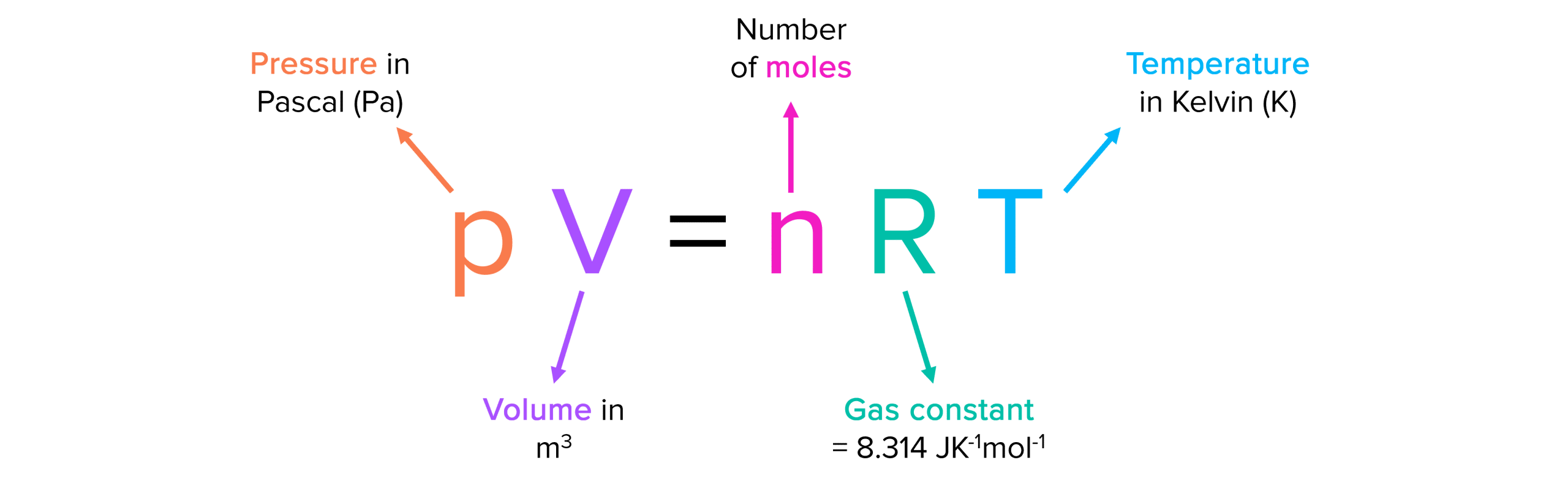Ideal Gas Equation Units . for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. pv = nrt (10.4.4) this equation is known as the ideal gas law. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Calculations using the ideal gas equation. The kinetic molecular theory of gases. An ideal gas is defined as a hypothetical gaseous substance whose. learn about the ideal gas law equation, its derivation, and its units. The ideal gas law (pv = nrt) what is the ideal gas law? the four gas variables are: learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass.
from
The ideal gas law (pv = nrt) what is the ideal gas law? for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. pv = nrt (10.4.4) this equation is known as the ideal gas law. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the four gas variables are: An ideal gas is defined as a hypothetical gaseous substance whose. Calculations using the ideal gas equation. learn about the ideal gas law equation, its derivation, and its units. The kinetic molecular theory of gases.
Ideal Gas Equation Units the four gas variables are: The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. learn about the ideal gas law equation, its derivation, and its units. Calculations using the ideal gas equation. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. The ideal gas law (pv = nrt) what is the ideal gas law? the four gas variables are: The kinetic molecular theory of gases. An ideal gas is defined as a hypothetical gaseous substance whose. pv = nrt (10.4.4) this equation is known as the ideal gas law.
From
Ideal Gas Equation Units for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. An ideal gas is defined as a hypothetical gaseous substance whose. The ideal gas law (pv = nrt) what is the ideal gas law? learn about the ideal gas law equation, its. Ideal Gas Equation Units.
From
Ideal Gas Equation Units pv = nrt (10.4.4) this equation is known as the ideal gas law. The ideal gas law (pv = nrt) what is the ideal gas law? Pressure (p), volume (v), number of mole of gas (n), and temperature (t). for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed. Ideal Gas Equation Units.
From www.slideserve.com
PPT Basic Laws of Gases and Particulates PowerPoint Presentation Ideal Gas Equation Units pv = nrt (10.4.4) this equation is known as the ideal gas law. The ideal gas law (pv = nrt) what is the ideal gas law? The kinetic molecular theory of gases. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). learn about the assumptions and units of the ideal gas law, and how. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The ideal gas law (pv = nrt) what is the ideal gas law? The kinetic molecular theory of gases. learn about the ideal gas law equation, its derivation, and its units. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. An. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). An ideal gas is defined as a hypothetical gaseous substance whose. the four gas variables are: The kinetic molecular theory of. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The kinetic molecular theory of gases. learn about the ideal gas law equation, its derivation, and its units. pv = nrt (10.4.4) this equation is known as the ideal gas law. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. An ideal. Ideal Gas Equation Units.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation Units learn about the ideal gas law equation, its derivation, and its units. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). learn about the assumptions and units of the. Ideal Gas Equation Units.
From
Ideal Gas Equation Units the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. The kinetic molecular theory of gases. The ideal gas law relates the pressure, volume,. Ideal Gas Equation Units.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy Ideal Gas Equation Units for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. the four gas variables are: learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. The kinetic. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The kinetic molecular theory of gases. the four gas variables are: pv = nrt (10.4.4) this equation is known as the ideal gas law. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. for example, in si units r = 8.3145. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. learn about the ideal gas law equation, its derivation, and its units. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals,. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The kinetic molecular theory of gases. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. An ideal gas is defined as a hypothetical gaseous substance whose. The ideal gas law (pv = nrt) what is the ideal gas law? The ideal gas. Ideal Gas Equation Units.
From www.youtube.com
LM Unit 9 Intro Ideal Gas Law YouTube Ideal Gas Equation Units The ideal gas law (pv = nrt) what is the ideal gas law? the four gas variables are: The kinetic molecular theory of gases. pv = nrt (10.4.4) this equation is known as the ideal gas law. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). learn about the assumptions and units of. Ideal Gas Equation Units.
From
Ideal Gas Equation Units Pressure (p), volume (v), number of mole of gas (n), and temperature (t). The ideal gas law (pv = nrt) what is the ideal gas law? The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. Calculations using the ideal gas equation. The kinetic molecular. Ideal Gas Equation Units.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Ideal Gas Equation Units for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. Calculations using the ideal gas equation. The kinetic molecular theory of gases. the four gas variables are: learn about the ideal gas law equation, its derivation, and its units. learn. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The ideal gas law (pv = nrt) what is the ideal gas law? learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. An ideal gas is defined as a hypothetical gaseous substance whose. pv = nrt (10.4.4) this equation is known as the. Ideal Gas Equation Units.
From
Ideal Gas Equation Units for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. An ideal gas is defined as a hypothetical. Ideal Gas Equation Units.
From
Ideal Gas Equation Units learn about the ideal gas law equation, its derivation, and its units. An ideal gas is defined as a hypothetical gaseous substance whose. The ideal gas law (pv = nrt) what is the ideal gas law? Pressure (p), volume (v), number of mole of gas (n), and temperature (t). learn about the assumptions and units of the ideal. Ideal Gas Equation Units.
From
Ideal Gas Equation Units pv = nrt (10.4.4) this equation is known as the ideal gas law. The ideal gas law (pv = nrt) what is the ideal gas law? the four gas variables are: learn about the ideal gas law equation, its derivation, and its units. for example, in si units r = 8.3145 j ⋅ k −1 ⋅. Ideal Gas Equation Units.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Ideal Gas Equation Units Calculations using the ideal gas equation. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. The kinetic molecular theory of gases. pv = nrt (10.4.4) this equation is known as the ideal gas law. An ideal gas is defined as a hypothetical gaseous. Ideal Gas Equation Units.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Ideal Gas Equation Units An ideal gas is defined as a hypothetical gaseous substance whose. learn about the ideal gas law equation, its derivation, and its units. Calculations using the ideal gas equation. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. for example, in si. Ideal Gas Equation Units.
From
Ideal Gas Equation Units Calculations using the ideal gas equation. The kinetic molecular theory of gases. learn about the ideal gas law equation, its derivation, and its units. The ideal gas law (pv = nrt) what is the ideal gas law? for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals,. Ideal Gas Equation Units.
From erebusryling.blogspot.com
Ideal Gas Law R Values PPT Gas Laws PowerPoint Presentation ID Ideal Gas Equation Units for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. The ideal gas law (pv = nrt) what is the ideal gas law? pv = nrt (10.4.4) this equation is known as the ideal gas law. learn about the ideal gas. Ideal Gas Equation Units.
From
Ideal Gas Equation Units An ideal gas is defined as a hypothetical gaseous substance whose. the four gas variables are: The kinetic molecular theory of gases. The ideal gas law (pv = nrt) what is the ideal gas law? pv = nrt (10.4.4) this equation is known as the ideal gas law. for example, in si units r = 8.3145 j. Ideal Gas Equation Units.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 Ideal Gas Equation Units pv = nrt (10.4.4) this equation is known as the ideal gas law. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in. Ideal Gas Equation Units.
From
Ideal Gas Equation Units learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. Calculations using the ideal gas equation. pv = nrt (10.4.4) this equation is known as the ideal gas law. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol. Ideal Gas Equation Units.
From
Ideal Gas Equation Units Pressure (p), volume (v), number of mole of gas (n), and temperature (t). The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and. Ideal Gas Equation Units.
From
Ideal Gas Equation Units pv = nrt (10.4.4) this equation is known as the ideal gas law. Calculations using the ideal gas equation. The kinetic molecular theory of gases. An ideal gas is defined as a hypothetical gaseous substance whose. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume. Ideal Gas Equation Units.
From www.grc.nasa.gov
Equation of State Ideal Gas Equation Units The ideal gas law (pv = nrt) what is the ideal gas law? pv = nrt (10.4.4) this equation is known as the ideal gas law. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. learn about the ideal gas law equation,. Ideal Gas Equation Units.
From
Ideal Gas Equation Units Pressure (p), volume (v), number of mole of gas (n), and temperature (t). An ideal gas is defined as a hypothetical gaseous substance whose. Calculations using the ideal gas equation. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. the four gas variables. Ideal Gas Equation Units.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation Units Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Calculations using the ideal gas equation. for example, in si units r = 8.3145 j ⋅ k −1 ⋅ mol −1 when pressure is expressed in pascals, volume in cubic meters, and. The ideal gas law relates the pressure, volume, temperature, and moles of a gas,. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The kinetic molecular theory of gases. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. for example, in. Ideal Gas Equation Units.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Ideal Gas Equation Units Calculations using the ideal gas equation. The kinetic molecular theory of gases. learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. learn about the ideal gas law equation, its derivation, and its units. Pressure (p), volume (v), number of mole of gas (n),. Ideal Gas Equation Units.
From
Ideal Gas Equation Units The ideal gas law (pv = nrt) what is the ideal gas law? An ideal gas is defined as a hypothetical gaseous substance whose. The ideal gas law relates the pressure, volume, temperature, and moles of a gas, and assumes that the gas particles are small, spherical, and elastic. Pressure (p), volume (v), number of mole of gas (n), and. Ideal Gas Equation Units.
From
Ideal Gas Equation Units learn about the ideal gas law equation, its derivation, and its units. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). learn about the assumptions and units of the ideal gas law, and how to use it to calculate molar volume and relative formula mass. An ideal gas is defined as a hypothetical gaseous. Ideal Gas Equation Units.