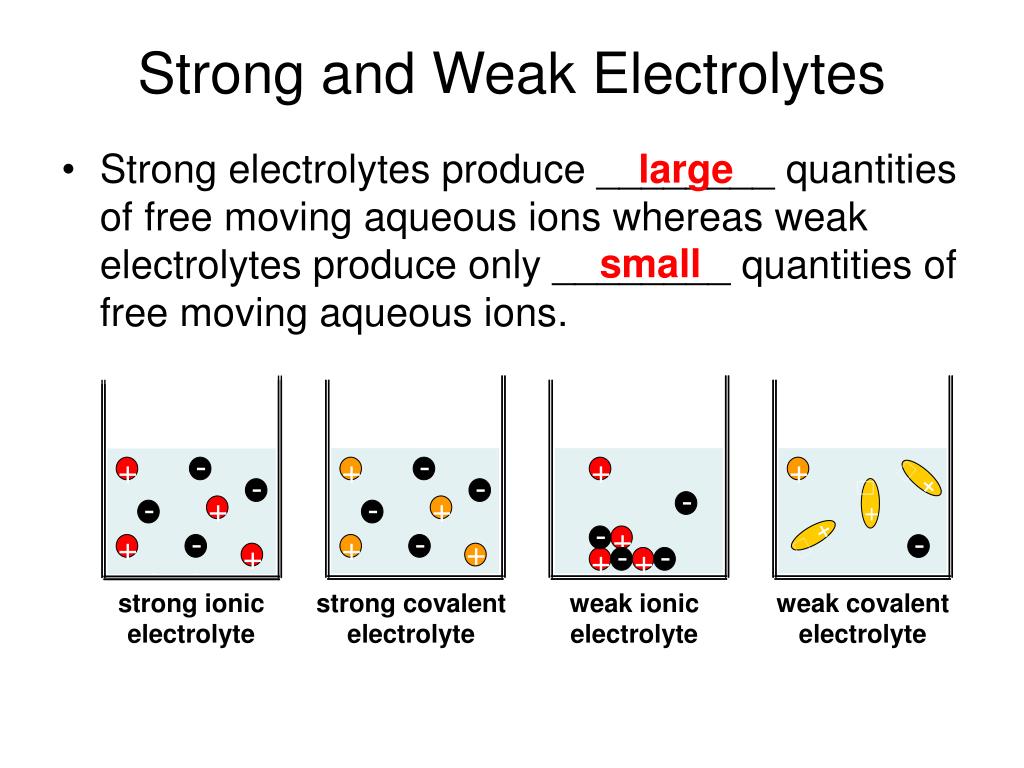
Strong Electrolyte Examples In Solution . A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Strong electrolytes break apart into ions. Define and give examples of electrolytes; Likewise, a strong acid like hcl splits. All soluble ionic compounds are strong electrolytes. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Distinguish between the physical and chemical changes that accompany dissolution of ionic. Strong electrolytes include the strong acids, strong bases, and salts. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. They conduct very well because they provide a plentiful supply of ions in solution. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. These chemicals completely dissociate into ions in.
from sharedocnow.blogspot.com
A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Strong electrolytes break apart into ions. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. These chemicals completely dissociate into ions in. They conduct very well because they provide a plentiful supply of ions in solution. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Define and give examples of electrolytes; Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. Distinguish between the physical and chemical changes that accompany dissolution of ionic. Strong electrolytes include the strong acids, strong bases, and salts.
List Of Strong Electrolytes Chemistry sharedoc
Strong Electrolyte Examples In Solution Strong electrolytes break apart into ions. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Strong electrolytes include the strong acids, strong bases, and salts. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Define and give examples of electrolytes; Likewise, a strong acid like hcl splits. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. Distinguish between the physical and chemical changes that accompany dissolution of ionic. These chemicals completely dissociate into ions in. All soluble ionic compounds are strong electrolytes. They conduct very well because they provide a plentiful supply of ions in solution. Strong electrolytes break apart into ions. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution.
From www.nagwa.com
Question Video Identifying Which of Three Substances Is a Strong Electrolyte Nagwa Strong Electrolyte Examples In Solution Distinguish between the physical and chemical changes that accompany dissolution of ionic. Likewise, a strong acid like hcl splits. Define and give examples of electrolytes; A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. All soluble ionic compounds are strong electrolytes. Strong electrolytes conduct electricity only in aqueous solutions, or in molten. Strong Electrolyte Examples In Solution.
From www.w3schools.blog
Strong and weak electrolytes W3schools Strong Electrolyte Examples In Solution These chemicals completely dissociate into ions in. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Strong electrolytes include the strong acids, strong bases, and salts. They conduct very well because they provide a plentiful supply of ions in. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID5584069 Strong Electrolyte Examples In Solution They conduct very well because they provide a plentiful supply of ions in solution. Define and give examples of electrolytes; Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. All soluble ionic compounds are strong electrolytes. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. These chemicals completely. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT SOLUTIONS OF ELECTROLYTES PowerPoint Presentation, free download ID817279 Strong Electrolyte Examples In Solution A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Define and give examples of electrolytes; A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Strong electrolytes include the strong acids, strong bases, and salts. All soluble ionic compounds are strong electrolytes. Strong electrolytes conduct electricity. Strong Electrolyte Examples In Solution.
From sharedocnow.blogspot.com
List Of Strong Electrolytes Chemistry sharedoc Strong Electrolyte Examples In Solution Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. All soluble ionic compounds are strong electrolytes. These chemicals completely dissociate into ions in. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. They conduct very well because they provide a plentiful supply of ions in. Strong Electrolyte Examples In Solution.
From www.shutterstock.com
19 Strong Electrolyte And Weak Electrolyte Images, Stock Photos & Vectors Shutterstock Strong Electrolyte Examples In Solution Define and give examples of electrolytes; Likewise, a strong acid like hcl splits. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. These chemicals completely dissociate into ions in. Strong electrolytes include the strong acids, strong bases, and salts. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry PowerPoint Presentation ID6291419 Strong Electrolyte Examples In Solution Define and give examples of electrolytes; They conduct very well because they provide a plentiful supply of ions in solution. These chemicals completely dissociate into ions in. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Strong electrolytes include the strong acids, strong bases, and salts. Likewise, a strong acid like. Strong Electrolyte Examples In Solution.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Strong Electrolyte Examples In Solution These chemicals completely dissociate into ions in. They conduct very well because they provide a plentiful supply of ions in solution. Define and give examples of electrolytes; A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Likewise, a strong acid like hcl splits. Strong electrolytes conduct electricity only in aqueous solutions,. Strong Electrolyte Examples In Solution.
From www.youtube.com
pH of a Strong Electrolyte (Example) YouTube Strong Electrolyte Examples In Solution A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Strong electrolytes include the strong acids, strong bases, and salts. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. All soluble ionic compounds are strong electrolytes. Strong electrolytes conduct electricity only in aqueous solutions, or in molten. Strong Electrolyte Examples In Solution.
From sharedocnow.blogspot.com
List Of Strong And Weak Electrolytes sharedoc Strong Electrolyte Examples In Solution These chemicals completely dissociate into ions in. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Define and give examples of electrolytes; Strong electrolytes include the strong acids, strong bases, and salts. Distinguish between the physical and chemical changes that accompany dissolution of ionic. A strong electrolyte, like nacl, splits up completely. Strong Electrolyte Examples In Solution.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes Chemistry Examples Strong Electrolyte Examples In Solution Distinguish between the physical and chemical changes that accompany dissolution of ionic. They conduct very well because they provide a plentiful supply of ions in solution. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. Define and give examples. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID9726136 Strong Electrolyte Examples In Solution Strong electrolytes break apart into ions. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. These chemicals completely dissociate into ions in. Define and give examples of electrolytes; They conduct very well because they provide a plentiful supply of ions in solution. Likewise, a strong acid like hcl splits. Distinguish between. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation ID7041191 Strong Electrolyte Examples In Solution A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. They conduct very well because they provide a plentiful supply of ions in solution. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. A strong electrolyte is a solute or solution that is an electrolyte that. Strong Electrolyte Examples In Solution.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Strong Electrolyte Examples In Solution All soluble ionic compounds are strong electrolytes. They conduct very well because they provide a plentiful supply of ions in solution. Strong electrolytes break apart into ions. Likewise, a strong acid like hcl splits. Strong electrolytes include the strong acids, strong bases, and salts. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. A. Strong Electrolyte Examples In Solution.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Strong Electrolyte Examples In Solution Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. They conduct very well because they provide a plentiful supply of ions in solution. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Likewise, a strong acid like hcl splits. All soluble ionic compounds are strong electrolytes. Define and. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chapter 9 Solutions PowerPoint Presentation, free download ID3593830 Strong Electrolyte Examples In Solution Strong electrolytes include the strong acids, strong bases, and salts. Strong electrolytes break apart into ions. All soluble ionic compounds are strong electrolytes. These chemicals completely dissociate into ions in. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. Define and give examples of electrolytes; Likewise, a strong acid like hcl splits. A strong. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free download ID4763576 Strong Electrolyte Examples In Solution Define and give examples of electrolytes; Distinguish between the physical and chemical changes that accompany dissolution of ionic. All soluble ionic compounds are strong electrolytes. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. These chemicals completely. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Lecture 7. Electrolytes. Reactions in Aqueous Solutions PowerPoint Presentation ID5813238 Strong Electrolyte Examples In Solution They conduct very well because they provide a plentiful supply of ions in solution. Define and give examples of electrolytes; Distinguish between the physical and chemical changes that accompany dissolution of ionic. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chapter 13 Ions in Aqueous Solutions and Colligative Properties PowerPoint Presentation Strong Electrolyte Examples In Solution A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. These chemicals completely dissociate into ions in. They conduct very well because they provide a plentiful supply of ions in solution. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. A strong electrolyte is a solution in which a. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3154052 Strong Electrolyte Examples In Solution Define and give examples of electrolytes; Likewise, a strong acid like hcl splits. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Strong electrolytes include the strong acids, strong bases, and salts. They conduct very well because they provide a plentiful supply of ions in solution. Strong electrolytes break apart into. Strong Electrolyte Examples In Solution.
From scottscience.weebly.com
Unit 11 Solutions Mr. Scott's Online Classroom Strong Electrolyte Examples In Solution A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. They conduct very well because they provide a plentiful supply of ions in solution. All soluble ionic compounds are strong electrolytes. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Strong electrolytes conduct electricity only in aqueous. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation ID159152 Strong Electrolyte Examples In Solution Strong electrolytes include the strong acids, strong bases, and salts. Likewise, a strong acid like hcl splits. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. All soluble ionic compounds are strong electrolytes. These chemicals completely dissociate into ions. Strong Electrolyte Examples In Solution.
From melissa-media.blogspot.com
Melissa Media Strong And Weak Electrolytes Strong Electrolyte Examples In Solution Distinguish between the physical and chemical changes that accompany dissolution of ionic. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. Strong electrolytes include the strong acids, strong bases, and salts. Define and give examples of electrolytes; All soluble ionic compounds are strong electrolytes. A strong electrolyte is a solute or solution that is. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I PowerPoint Presentation Strong Electrolyte Examples In Solution Strong electrolytes include the strong acids, strong bases, and salts. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. These chemicals completely dissociate into ions in. Distinguish between the physical and chemical changes that accompany dissolution of. Strong Electrolyte Examples In Solution.
From www.kentchemistry.com
Electrolytes Strong Electrolyte Examples In Solution These chemicals completely dissociate into ions in. They conduct very well because they provide a plentiful supply of ions in solution. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. A strong electrolyte, like. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID442177 Strong Electrolyte Examples In Solution Define and give examples of electrolytes; Likewise, a strong acid like hcl splits. All soluble ionic compounds are strong electrolytes. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Distinguish between the physical and. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID6630187 Strong Electrolyte Examples In Solution Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. Strong electrolytes break apart into ions. Define and give examples of electrolytes; Likewise, a strong acid like hcl splits. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. A strong electrolyte, like nacl, splits up completely. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free download ID4763570 Strong Electrolyte Examples In Solution Distinguish between the physical and chemical changes that accompany dissolution of ionic. Strong electrolytes include the strong acids, strong bases, and salts. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. A strong electrolyte,. Strong Electrolyte Examples In Solution.
From slideserve.com
PPT II. Types of Electrolytes PowerPoint Presentation ID297972 Strong Electrolyte Examples In Solution Likewise, a strong acid like hcl splits. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Distinguish between the physical and chemical changes that accompany dissolution of ionic. These chemicals completely dissociate into ions in. They conduct very well because they provide a plentiful supply of ions in solution. Strong electrolytes break. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free download ID1242210 Strong Electrolyte Examples In Solution Distinguish between the physical and chemical changes that accompany dissolution of ionic. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Likewise, a strong acid like hcl splits. All soluble ionic compounds are strong electrolytes. Strong electrolytes include the strong acids, strong bases, and salts. Strong electrolytes break apart into ions. Strong. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation ID5407668 Strong Electrolyte Examples In Solution A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Strong electrolytes include the strong acids, strong bases, and salts. Define and give examples of electrolytes; They conduct very well because they provide a plentiful supply of ions in solution. Distinguish between the physical and chemical changes that accompany dissolution of ionic. Strong electrolytes conduct. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Electrical conductivity of electrolyte’s solutions PowerPoint Presentation ID1381064 Strong Electrolyte Examples In Solution Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Likewise, a strong acid like hcl splits. Distinguish between the physical and chemical changes that accompany dissolution of ionic. A strong electrolyte is a solute or solution that is an. Strong Electrolyte Examples In Solution.
From www.numerade.com
SOLVEDWhat is meant by a strong electrolyte? Give two examples of substances that behave in Strong Electrolyte Examples In Solution Strong electrolytes include the strong acids, strong bases, and salts. Distinguish between the physical and chemical changes that accompany dissolution of ionic. All soluble ionic compounds are strong electrolytes. Strong electrolytes break apart into ions. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Strong electrolytes conduct electricity only in aqueous solutions, or in. Strong Electrolyte Examples In Solution.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Strong Electrolyte Examples In Solution They conduct very well because they provide a plentiful supply of ions in solution. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. All soluble ionic compounds are strong electrolytes. Strong electrolytes conduct electricity only in aqueous solutions, or in molten salt, and ionic liquid. Distinguish between the physical and chemical changes that accompany. Strong Electrolyte Examples In Solution.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download ID3760667 Strong Electrolyte Examples In Solution Likewise, a strong acid like hcl splits. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. All soluble ionic compounds are strong electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic. These chemicals completely dissociate into ions in. A strong electrolyte is a solution in which a large. Strong Electrolyte Examples In Solution.