Calculate The E.m.f Of The Cell . The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The fundamental definition is the number of joules of energy each coulomb of charge picks up as. There are two main equations used to calculate emf. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential difference is caused by the ability of. Fe fe a 2 + + 2 e a −. The formula for calculating the emf. Emf is measure by combining two separate half cells.
from askfilo.com
The formula for calculating the emf. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. There are two main equations used to calculate emf. The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. The potential difference is caused by the ability of. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. Fe fe a 2 + + 2 e a −. Emf is measure by combining two separate half cells. The fundamental definition is the number of joules of energy each coulomb of charge picks up as.
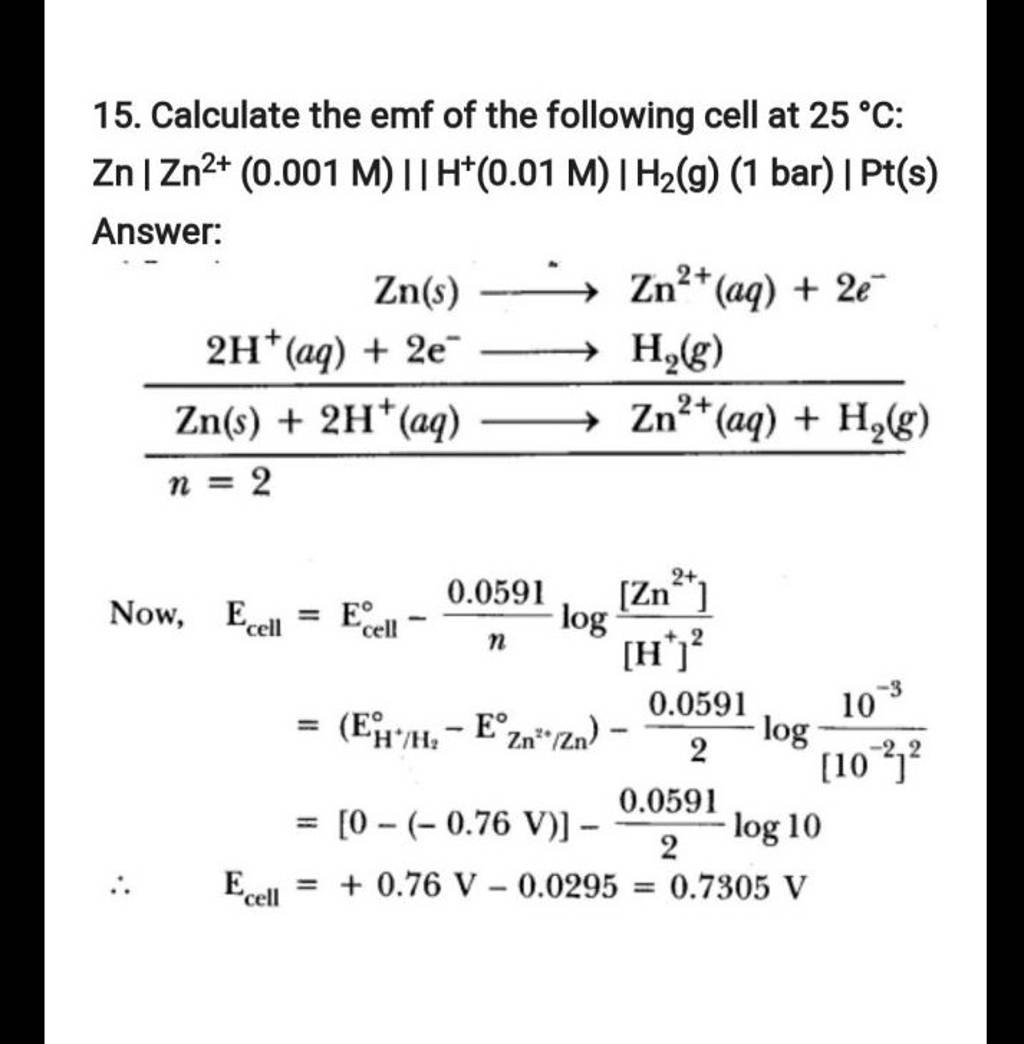
15. Calculate the emf of the following cell at 25∘C Zn∣∣ Zn2+(0.001M)∣∣..
Calculate The E.m.f Of The Cell The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. There are two main equations used to calculate emf. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The formula for calculating the emf. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The fundamental definition is the number of joules of energy each coulomb of charge picks up as. Emf is measure by combining two separate half cells. The potential difference is caused by the ability of. Fe fe a 2 + + 2 e a −. The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell.
From www.youtube.com
Write the nearest equation and calculate the e.m.f. of the following Calculate The E.m.f Of The Cell The formula for calculating the emf. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. The fundamental definition is the number of joules of energy each coulomb of charge picks up as. Emf is measure by combining two separate half cells. Fe (s) | fe. Calculate The E.m.f Of The Cell.
From askfilo.com
2. Calculate the e.m.f. of the cell in which the reaction is \[\begin{.. Calculate The E.m.f Of The Cell The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The potential difference is caused by the ability. Calculate The E.m.f Of The Cell.
From askfilo.com
2. Calculate the emf of the cell, Cd/Cd2+(0.001M)∣∣Fe2+(0.6M)∣Fe at 25∘C.. Calculate The E.m.f Of The Cell The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. The emf (electromotive force) of a cell is the maximum potential difference between. Calculate The E.m.f Of The Cell.
From brainly.in
Calculate the emf of the following cell at 298K. Mg(s)/Mg2+(0.1M Calculate The E.m.f Of The Cell The fundamental definition is the number of joules of energy each coulomb of charge picks up as. The potential difference is caused by the ability of. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential, d eo, of a galvanic cell can be evaluated. Calculate The E.m.f Of The Cell.
From byjus.com
Write Nernst equation and calculate e.m.f. of the following cell at 298 Calculate The E.m.f Of The Cell The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q). Calculate The E.m.f Of The Cell.
From www.toppr.com
Calculate e.m.f of the following cell at 298 K 2Cr (s) + 3Fe^2 + (0 Calculate The E.m.f Of The Cell The formula for calculating the emf. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. There are two main equations used to calculate emf. Emf. Calculate The E.m.f Of The Cell.
From askfilo.com
ΔG∘=−43600 J at 25∘C. Calculate the e.m.f. of the cell. (log10−n=−n) (3/5.. Calculate The E.m.f Of The Cell Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. Fe fe a 2 + + 2 e. Calculate The E.m.f Of The Cell.
From askfilo.com
16 Calculate the emf of the cell in which the following reaction takes p.. Calculate The E.m.f Of The Cell Emf is measure by combining two separate half cells. The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The maximum potential difference which can be measured for a. Calculate The E.m.f Of The Cell.
From www.doubtnut.com
Calculate the e.m.f. of the cell, Cr//Cr^(3+)(0.1 M) Fe^(2+)(0.01 Calculate The E.m.f Of The Cell The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The potential difference is caused by the ability of. Fe fe. Calculate The E.m.f Of The Cell.
From www.toppr.com
Calculate the EMF of the cell Fe(s) + 2H+ (1 M) Fe+2 (0.001 M) + H2(g Calculate The E.m.f Of The Cell The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. Fe fe a 2 + + 2 e a −. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. The potential difference. Calculate The E.m.f Of The Cell.
From askfilo.com
(a) Calculate the emf of the following cell at 298 K.\[\begin{array}{l}.. Calculate The E.m.f Of The Cell Emf is measure by combining two separate half cells. The potential difference is caused by the ability of. Fe fe a 2 + + 2 e a −. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The emf or electromotive force is the energy supplied by a battery. Calculate The E.m.f Of The Cell.
From www.toppr.com
30. Calculate the e.m.f. of the following cell 298 K. Sn (s) Sn+2 (0.05 Calculate The E.m.f Of The Cell The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The formula for calculating the emf. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Fe (s) | fe 2+ (0.01 m) | | h. Calculate The E.m.f Of The Cell.
From quizpersonable.z4.web.core.windows.net
Calculate The Standard Emf Of The Cell Calculate The E.m.f Of The Cell The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar). Calculate The E.m.f Of The Cell.
From brainly.in
Write the nerst equation and calculate the e.m.f of following cell at Calculate The E.m.f Of The Cell The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The formula for calculating the emf. There are two main equations used to calculate emf. Fe fe a 2 + + 2 e a −. The fundamental definition is the number of joules of energy each coulomb of charge. Calculate The E.m.f Of The Cell.
From www.numerade.com
SOLVED Calculate the emf of the following cell at 25 °C Zn Zn2+ (0 Calculate The E.m.f Of The Cell The fundamental definition is the number of joules of energy each coulomb of charge picks up as. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. Emf is measure by combining two separate half cells. The cell potential, \ (e_ {cell}\), is the measure of. Calculate The E.m.f Of The Cell.
From askfilo.com
2. Calculate the e.m.f. of the cell in which the reaction is \[ \begin{.. Calculate The E.m.f Of The Cell Fe fe a 2 + + 2 e a −. The potential difference is caused by the ability of. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s). Calculate The E.m.f Of The Cell.
From www.doubtnut.com
[Punjabi] Calculate the e.m.f. of the cell at 25^()C CrCr^(3+)(0.1M) Calculate The E.m.f Of The Cell The fundamental definition is the number of joules of energy each coulomb of charge picks up as. Emf is measure by combining two separate half cells. The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. Fe fe a 2 + + 2 e a −. The cell potential, \ (e_ {cell}\),. Calculate The E.m.f Of The Cell.
From askfilo.com
AII Q.7. (a) Write the cell reaction and calculate the e.m.f. of the foll.. Calculate The E.m.f Of The Cell The fundamental definition is the number of joules of energy each coulomb of charge picks up as. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The potential difference is caused by the ability of. The standard cell potential, d eo,. Calculate The E.m.f Of The Cell.
From www.numerade.com
SOLVED Calculate the EMF of a Danial cell when the concentration of Calculate The E.m.f Of The Cell The fundamental definition is the number of joules of energy each coulomb of charge picks up as. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The formula for calculating the emf. The potential difference is caused by the ability of.. Calculate The E.m.f Of The Cell.
From www.toppr.com
2) Calculate the emf of the cell. Zn Zn2+ (0.008M) Cr3+ (0.01M) Cr Calculate The E.m.f Of The Cell The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The emf or electromotive force is the energy supplied by a battery or a. Calculate The E.m.f Of The Cell.
From askfilo.com
12. Calculate e.m.f of the following cell Zn(s) ∣∣ Zn2+(0.1M)∥Ag+(0.01M.. Calculate The E.m.f Of The Cell Emf is measure by combining two separate half cells. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf (electromotive force) of a. Calculate The E.m.f Of The Cell.
From www.coursehero.com
[Solved] Calculate the emf of a cell using the Nernst equation Zn/Zn Calculate The E.m.f Of The Cell The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Emf. Calculate The E.m.f Of The Cell.
From www.doubtnut.com
Calculate the e.m.f. of the cell, Mg//Mg^(2+)(0.1 M)Ag^(+)(1.0xx10 Calculate The E.m.f Of The Cell There are two main equations used to calculate emf. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential. Calculate The E.m.f Of The Cell.
From www.youtube.com
Calculate the e.m.f of the following cell CdCd^(2+) (0.01 M) H Calculate The E.m.f Of The Cell The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The formula for calculating the emf. The fundamental definition is the number of joules of energy each. Calculate The E.m.f Of The Cell.
From askfilo.com
15. Calculate the emf of the following cell at 25∘C Zn∣∣ Zn2+(0.001M)∣∣.. Calculate The E.m.f Of The Cell Fe fe a 2 + + 2 e a −. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. Fe (s) | fe 2+ (0.01 m) | | h + (1. Calculate The E.m.f Of The Cell.
From www.toppr.com
Calculate e.m.f of the following cell at 298 K 2Cr (s) + 3Fe^2 + (0 Calculate The E.m.f Of The Cell Emf is measure by combining two separate half cells. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The fundamental definition is the number of joules of energy. Calculate The E.m.f Of The Cell.
From www.doubtnut.com
Calculate the EMF of the cell for the reaction. Mg((s))+2Ag((aq))^+ Mg Calculate The E.m.f Of The Cell The fundamental definition is the number of joules of energy each coulomb of charge picks up as. Fe fe a 2 + + 2 e a −. The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. The standard cell potential, d eo, of a galvanic cell can be evaluated. Calculate The E.m.f Of The Cell.
From www.meritnation.com
Calculate the emf of the cell and write the cell reaction Cr/Cr3+(0 1M Calculate The E.m.f Of The Cell The formula for calculating the emf. Emf is measure by combining two separate half cells. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half. Calculate The E.m.f Of The Cell.
From www.toppr.com
Write the cell reaction and calculate the e.m.f. of the following cell Calculate The E.m.f Of The Cell The formula for calculating the emf. The potential difference is caused by the ability of. The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. Emf is measure by combining two separate half cells. The emf (electromotive force) of a cell is the maximum potential difference. Calculate The E.m.f Of The Cell.
From www.youtube.com
Calculate e.m.f of the following cell Zn(s)/Zn2+ (0.1 M) (0.01 M Calculate The E.m.f Of The Cell The standard cell potential, d eo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells eo. Emf is measure by combining two separate half cells. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. There are two main equations used. Calculate The E.m.f Of The Cell.
From askfilo.com
Numericals1) Calculate the emf of the cell in which the following reacti.. Calculate The E.m.f Of The Cell The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. The maximum potential difference which can be measured for a given cell is called the electromotive. Calculate The E.m.f Of The Cell.
From www.toppr.com
1. Calculate the emf of the following cell 298K . Mg/Mg2+(0.001M)// Cu Calculate The E.m.f Of The Cell The emf or electromotive force is the energy supplied by a battery or a cell per coulomb (q) of charge passing through it. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The potential difference is caused by the ability of.. Calculate The E.m.f Of The Cell.
From brainly.in
(a) Calculate the emf of the following cell at 25°C Zn (s) Zn²+ (0.1 M Calculate The E.m.f Of The Cell Emf is measure by combining two separate half cells. Fe (s) | fe 2+ (0.01 m) | | h + (1 m) | h 2 (g) (1 bar) pt (s) e a cell 0 = 0.44 v. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf. Calculate The E.m.f Of The Cell.
From www.toppr.com
3.27 Calculate the e.m.f. of the following cell 298 K Mg Mg2+ (0.001 Calculate The E.m.f Of The Cell The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf (electromotive force) of a cell is the maximum potential difference between two electrodes of a cell. Fe fe a 2 + + 2 e a −. There are two main equations used to calculate emf. The fundamental. Calculate The E.m.f Of The Cell.
From www.toppr.com
Write the cell reaction and calculate the e.m.f. of the following cell Calculate The E.m.f Of The Cell The maximum potential difference which can be measured for a given cell is called the electromotive force, abbreviated emf and. Emf is measure by combining two separate half cells. The potential difference is caused by the ability of. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Fe. Calculate The E.m.f Of The Cell.