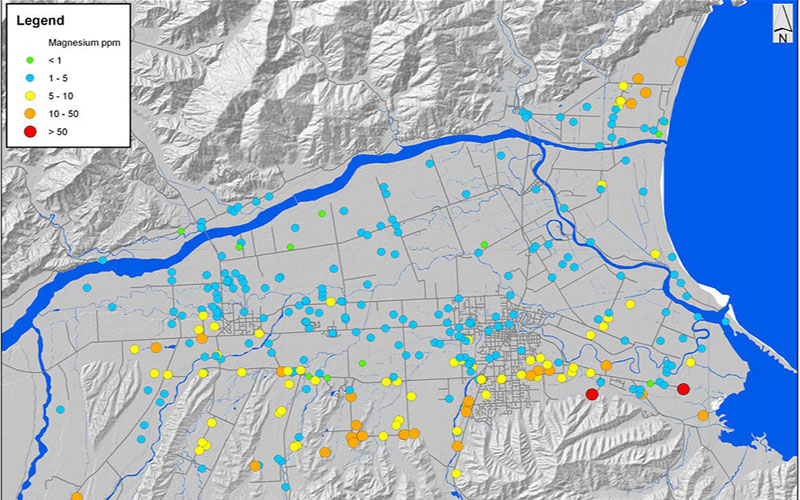
Magnesium Groundwater . Magnesium is present in large quantities in sea water. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Including positively charged ions called cations (sodium, na +; In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. The six constituents typically present at highest concentrations in groundwater are known as major ions: Water low in calcium and magnesium is desired in.
from www.marlborough.govt.nz
Magnesium is present in large quantities in sea water. Water low in calcium and magnesium is desired in. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Including positively charged ions called cations (sodium, na +; Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. The six constituents typically present at highest concentrations in groundwater are known as major ions: Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved.
Magnesium Marlborough District Council
Magnesium Groundwater Water low in calcium and magnesium is desired in. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Water low in calcium and magnesium is desired in. The six constituents typically present at highest concentrations in groundwater are known as major ions: Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Magnesium is present in large quantities in sea water. Including positively charged ions called cations (sodium, na +;
From www.researchgate.net
Spatial variation for Magnesium Adsorption Ratio during four Magnesium Groundwater Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium is present in large quantities in sea water. Water low in calcium and magnesium is desired in. Including positively charged ions. Magnesium Groundwater.
From www.scribd.com
WST S 09 01021 PDF Magnesium Groundwater Magnesium Groundwater In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Including positively charged ions called cations (sodium, na +; Magnesium is present in large quantities in sea water. The six constituents typically present at highest concentrations in groundwater are known as major ions: Due to the differences in water rock interaction, the magnesium isotope. Magnesium Groundwater.
From www.researchgate.net
Variation of magnesium hazard values of groundwater at sampling Magnesium Groundwater Magnesium is present in large quantities in sea water. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Including positively charged ions called cations (sodium, na +; In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium naturally occurring in water. Magnesium Groundwater.
From www.scribd.com
TMP 2 F56 PDF Magnesium Groundwater Magnesium Groundwater Magnesium is present in large quantities in sea water. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Water low in calcium and magnesium is desired in. Including positively charged ions called cations (sodium, na +; In scientific terms, water hardness is generally the amount of dissolved calcium. Magnesium Groundwater.
From www.researchgate.net
(PDF) Granular Matrix Supported Nano Magnesium Oxide for Groundwater Magnesium Groundwater The six constituents typically present at highest concentrations in groundwater are known as major ions: Including positively charged ions called cations (sodium, na +; In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Magnesium is present. Magnesium Groundwater.
From www.scribd.com
Ground Water Information Booklet of West District, NCT, Delhi PDF Magnesium Groundwater Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Water low in calcium and magnesium is desired in. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. In scientific terms, water hardness is generally the amount of dissolved calcium. Magnesium Groundwater.
From www.researchgate.net
Magnesium Content in Groundwater of Amritsar District (Acceptable limit Magnesium Groundwater Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Including positively charged ions called. Magnesium Groundwater.
From www.marlborough.govt.nz
Magnesium Marlborough District Council Magnesium Groundwater Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Magnesium is present in large quantities in sea water. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Water low in calcium and magnesium is desired in. Including positively charged ions called. Magnesium Groundwater.
From www.scribd.com
Hydrochemistry of groundwater in North Rajasthan, India PDF Magnesium Groundwater Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Water low in calcium and magnesium is desired in. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils. Magnesium Groundwater.
From shopee.com.my
Dolomite (Ground Magnesium Limestone) GML for Gardening and Agriculture Magnesium Groundwater Including positively charged ions called cations (sodium, na +; Water low in calcium and magnesium is desired in. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Magnesium is present in large quantities in sea water. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different. Magnesium Groundwater.
From www.researchgate.net
Magnesium hazard (MH), SAR, TDS, EC and Na values in the wells of the Magnesium Groundwater Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Magnesium is present in large quantities in sea water. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. The six constituents typically present at highest concentrations in groundwater are known as. Magnesium Groundwater.
From www.slideserve.com
PPT Hard Water & Soft Water PowerPoint Presentation ID2271804 Magnesium Groundwater Including positively charged ions called cations (sodium, na +; In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. The six constituents typically present at highest concentrations in groundwater are known as major ions: Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain. Magnesium Groundwater.
From water.unl.edu
Naturally Occurring Elements in Nebraska’s Groundwater Part 1 of a Magnesium Groundwater Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Including positively charged ions called cations (sodium, na +; Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Water low in calcium and magnesium is desired in. Magnesium is present in. Magnesium Groundwater.
From fcit.usf.edu
Concentrations of Calcium, Magnesium, and Sodium in Groundwater from Magnesium Groundwater In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Water low in calcium and magnesium is desired in. Including positively charged ions called cations (sodium, na +; Due to the differences in water rock interaction, the. Magnesium Groundwater.
From pubs.sciepub.com
Figure 7. Distribution of Magnesium in shallow and deeper aquifers Magnesium Groundwater Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Water low in calcium and magnesium is desired in. The six constituents typically present at highest concentrations in groundwater are known as. Magnesium Groundwater.
From www.scribd.com
Delineation of Groundwater Potential Zones and Zones of Groundwater Magnesium Groundwater Including positively charged ions called cations (sodium, na +; Water low in calcium and magnesium is desired in. The six constituents typically present at highest concentrations in groundwater are known as major ions: Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Water is a solvent and dissolves minerals from the rocks. Magnesium Groundwater.
From en.earth-science.net
Geological Genesis of Alkaline MagnesiumType Groundwater within the Magnesium Groundwater Including positively charged ions called cations (sodium, na +; The six constituents typically present at highest concentrations in groundwater are known as major ions: In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium is present in large quantities in sea water. Magnesium naturally occurring in water as a result of groundwater dissolving. Magnesium Groundwater.
From books.gw-project.org
Natural Chemical Constituents in Groundwater Groundwater in Our Water Magnesium Groundwater Water low in calcium and magnesium is desired in. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Including positively charged ions called cations (sodium, na +; Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. The six constituents typically present. Magnesium Groundwater.
From www.researchgate.net
Variation of magnesium hazard values of groundwater at sampling Magnesium Groundwater Including positively charged ions called cations (sodium, na +; Magnesium is present in large quantities in sea water. Water low in calcium and magnesium is desired in. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Magnesium naturally occurring in water as a result of groundwater dissolving. Magnesium Groundwater.
From www.researchgate.net
Hydrochemical indicators of groundwater samplings (a) magnesium (Mg Magnesium Groundwater The six constituents typically present at highest concentrations in groundwater are known as major ions: Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Magnesium is present in large quantities in sea water. Including positively charged ions called cations (sodium, na +; Water is a solvent and. Magnesium Groundwater.
From www.youtube.com
Magnesium Ground State Electron Configuration YouTube Magnesium Groundwater Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Magnesium is present in large quantities in sea water. Magnesium naturally occurring in water as a result. Magnesium Groundwater.
From www.researchgate.net
(PDF) Magnesium and groundwater flow relationship in karst aquifers a Magnesium Groundwater Magnesium is present in large quantities in sea water. Water low in calcium and magnesium is desired in. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. The six constituents typically present at highest concentrations in groundwater are known as major ions: Due to the differences in water rock interaction, the magnesium isotope. Magnesium Groundwater.
From www.researchgate.net
Spatial distribution of groundwater quality parameters A) Magnesium, B Magnesium Groundwater Magnesium is present in large quantities in sea water. Water low in calcium and magnesium is desired in. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. The six constituents typically present at highest concentrations in. Magnesium Groundwater.
From www.lazada.com.my
GROUND MAGNESIUM LIMESTONE 2KG Kapur Dolomite 15 Magnesium Oksida(MgO Magnesium Groundwater The six constituents typically present at highest concentrations in groundwater are known as major ions: Water low in calcium and magnesium is desired in. Magnesium is present in large quantities in sea water. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Due to the differences in water. Magnesium Groundwater.
From www.researchgate.net
c. Areal distribution of dissolved magnesium in groundwater (in mg/l Magnesium Groundwater The six constituents typically present at highest concentrations in groundwater are known as major ions: Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Including positively charged ions called cations (sodium,. Magnesium Groundwater.
From pubs.sciepub.com
Figure 8. Distribution of Magnesium in shallow and deeper aquifers Magnesium Groundwater The six constituents typically present at highest concentrations in groundwater are known as major ions: Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Water is a solvent and dissolves minerals. Magnesium Groundwater.
From www.researchgate.net
(PDF) Defluoridation of groundwater using magnesium oxide Magnesium Groundwater In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Including positively charged ions called cations (sodium, na +; Water low in calcium and magnesium is desired in. The six constituents typically present at highest concentrations in groundwater are known as major ions: Due to the differences in water rock interaction, the magnesium isotope. Magnesium Groundwater.
From www.researchgate.net
Spatial variation of magnesium in groundwater Download Scientific Diagram Magnesium Groundwater Magnesium is present in large quantities in sea water. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Water low in calcium and magnesium is desired in. Including positively charged ions called cations (sodium, na +; Magnesium naturally occurring in water as a result of groundwater dissolving. Magnesium Groundwater.
From www.researchgate.net
Hydrochemical facies of groundwater. A—Calcium type, B—No Dominant Magnesium Groundwater The six constituents typically present at highest concentrations in groundwater are known as major ions: In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Including positively charged ions called cations (sodium,. Magnesium Groundwater.
From www.semanticscholar.org
Figure 1 from Magnesium and groundwater flow relationship in karst Magnesium Groundwater Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Magnesium is present in large quantities in sea water. Water low in calcium and magnesium is desired in. The six constituents typically present. Magnesium Groundwater.
From www.scribd.com
Groundwater Quality Assessment For Drin PDF Water Quality Magnesium Magnesium Groundwater Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Water low in calcium and magnesium is desired in. Including positively charged ions called cations (sodium, na +; Water is a. Magnesium Groundwater.
From www.researchgate.net
(PDF) Impact of Calcium and Magnesium in Groundwater and Drinking Water Magnesium Groundwater Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Including positively charged ions called cations (sodium, na +; Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. The six constituents typically present at highest concentrations in groundwater are known as. Magnesium Groundwater.
From www.researchgate.net
Magnesium distribution in groundwater of the area Download Scientific Magnesium Groundwater Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock.. Magnesium Groundwater.
From www.researchgate.net
Distribution of magnesium ion in groundwater in the Narpay Canal Basin Magnesium Groundwater Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Water low in calcium and magnesium is desired in. Magnesium is present in large quantities in sea water. Magnesium naturally occurring in water as a result of groundwater dissolving magnesium from soils or dolomite rock. In scientific terms,. Magnesium Groundwater.
From www.scribd.com
Assessment of Water Quality Using GIS PDF Magnesium Groundwater Magnesium Groundwater Water is a solvent and dissolves minerals from the rocks with which it comes in contact, so groundwater may contain dissolved. In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. Due to the differences in water rock interaction, the magnesium isotope composition of groundwater in different types of aquifers has their own. Including. Magnesium Groundwater.