The Cooling System Of A Car Engine Contains 20.0 . To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). (1 l of water has a mass of 1 kg.) what is the change in the temperature. The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). The cooling system of a car engine contains 20.0 l of water. To find the change in temperature of the water, we can use the formula: What is the change of temperature in the water if the. A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! First, we need to use the specific heat capacity of water to calculate the change in temperature. Which coolant, water or methanol, would better. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). What is the change in temperature of.
from consumera.com
To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. What is the change in temperature of. To find the change in temperature of the water, we can use the formula: The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). What is the change of temperature in the water if the. (1 l of water has a mass of 1 kg.) what is the change in the temperature. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). Which coolant, water or methanol, would better. A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go!
How Does A Car's Cooling System Work?
The Cooling System Of A Car Engine Contains 20.0 The cooling system of a car engine contains 20.0 l of water. (1 l of water has a mass of 1 kg.) what is the change in the temperature. What is the change of temperature in the water if the. Which coolant, water or methanol, would better. The cooling system of a car engine contains 20.0 l of water. First, we need to use the specific heat capacity of water to calculate the change in temperature. A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). To find the change in temperature of the water, we can use the formula: To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. What is the change in temperature of.
From thecarclinicofmiami.com
The Basics of Car Cooling System Maintenance The Car Clinic of Miami The Cooling System Of A Car Engine Contains 20.0 To find the change in temperature of the water, we can use the formula: A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1. The Cooling System Of A Car Engine Contains 20.0.
From www.dubizzle.com
Types of Engine Cooling Systems Working, Pros & More The Cooling System Of A Car Engine Contains 20.0 What is the change of temperature in the water if the. Which coolant, water or methanol, would better. The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). To find the change in temperature of the water, we can use the formula: The cooling system of a car. The Cooling System Of A Car Engine Contains 20.0.
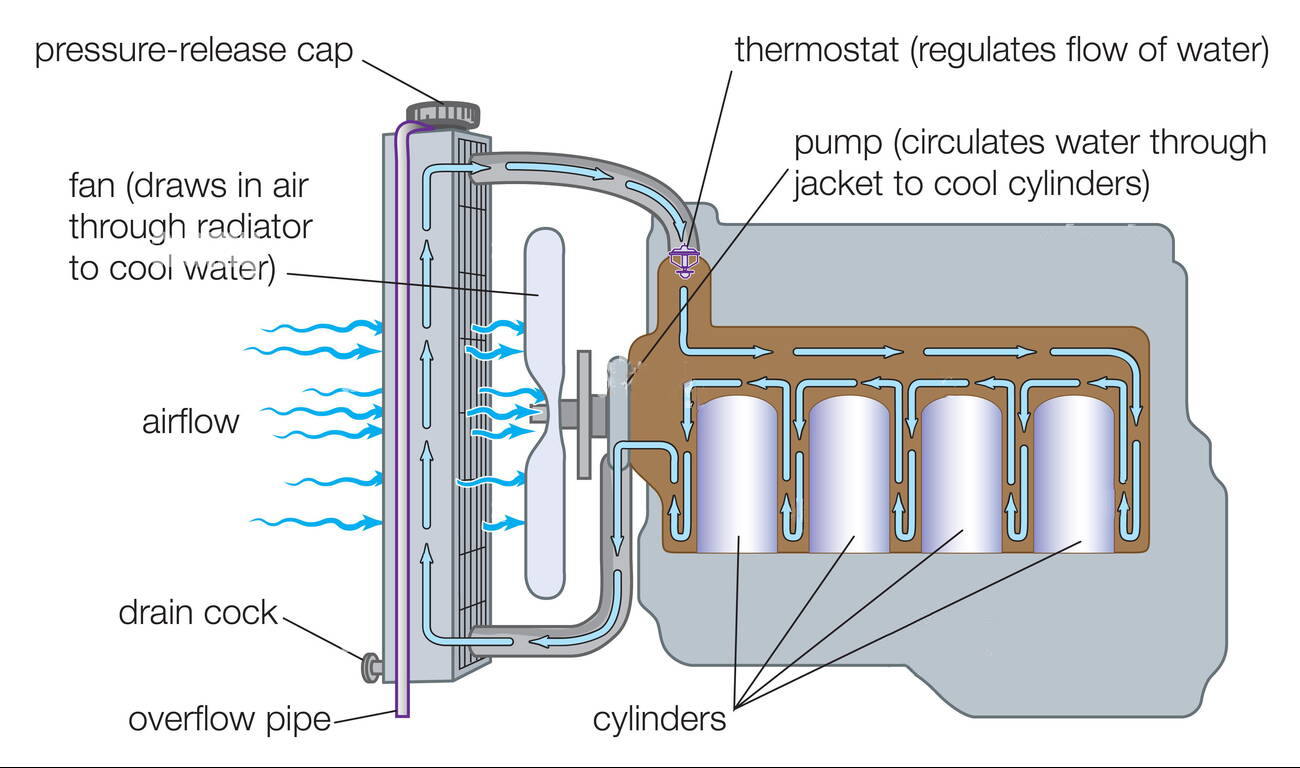
From www.howacarworks.com
How an engine cooling system works How a Car Works The Cooling System Of A Car Engine Contains 20.0 The cooling system of a car engine contains 20.0 l of water. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). First, we need to use the specific heat capacity of water to calculate the change in temperature. To determine the change in temperature of the. The Cooling System Of A Car Engine Contains 20.0.
From www.howacarworks.com
How an engine cooling system works How a Car Works The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). The cooling system of a car engine contains 20.0 l of water. Which coolant, water or methanol, would better. To find the change in temperature of the water, we can use the formula: What is the change in. The Cooling System Of A Car Engine Contains 20.0.
From toytechs.com
What you need to know about your Automotive Cooling System TOYTECHS The Cooling System Of A Car Engine Contains 20.0 To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). First, we need to use the specific heat capacity of water to calculate the change in temperature.. The Cooling System Of A Car Engine Contains 20.0.
From engineeringdiscoveries.com
How Engine Cooling System Works? Engineering Discoveries The Cooling System Of A Car Engine Contains 20.0 To find the change in temperature of the water, we can use the formula: Which coolant, water or methanol, would better. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). To determine the change in temperature of the water in the car engine's cooling system, we. The Cooling System Of A Car Engine Contains 20.0.
From www.counterman.com
A Closer Look At Engine Cooling Components The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). What is the change in temperature of. To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. (1 l of water has a mass of 1 kg.). The Cooling System Of A Car Engine Contains 20.0.
From www.youtube.com
How Car Cooling System Works YouTube The Cooling System Of A Car Engine Contains 20.0 What is the change in temperature of. The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). (1 l of water has a mass of 1 kg.) what is the change in the temperature. First, we need to use the specific heat capacity of water to calculate the. The Cooling System Of A Car Engine Contains 20.0.
From engineeringdiscoveries.com
How Engine Cooling System Works? Engineering Discoveries The Cooling System Of A Car Engine Contains 20.0 First, we need to use the specific heat capacity of water to calculate the change in temperature. Which coolant, water or methanol, would better. To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. (1 l of water has a mass of 1 kg.) what is the change in. The Cooling System Of A Car Engine Contains 20.0.
From schempal.com
The Ultimate Guide to Understanding the Dodge Journey Cooling System The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). What is the change of temperature in the water if the. (1 l of water has a mass of 1 kg.) what is the change in the temperature. A car engine's cooling system contains 20.0 l of water. The Cooling System Of A Car Engine Contains 20.0.
From www.youtube.com
Animation on How Vehicle Cooling Systems Work YouTube The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! Which coolant, water or methanol, would better. The cooling system of a car engine contains 20.0 l of water. (1 l of water has a mass of 1 kg.) what is the change in the. The Cooling System Of A Car Engine Contains 20.0.
From www.carparts.com
Automotive Cooling Systems A Short Course on How They Work The Cooling System Of A Car Engine Contains 20.0 What is the change in temperature of. (1 l of water has a mass of 1 kg.) what is the change in the temperature. The cooling system of a car engine contains 20.0 l of water. To find the change in temperature of the water, we can use the formula: To determine the change in temperature of the water in. The Cooling System Of A Car Engine Contains 20.0.
From www.newkidscar.com
How Engine Cooling System works Car Anatomy The Cooling System Of A Car Engine Contains 20.0 The cooling system of a car engine contains 20.0 l of water. To find the change in temperature of the water, we can use the formula: The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). A car engine's cooling system contains 20.0 l of water ( 1. The Cooling System Of A Car Engine Contains 20.0.
From www.mycarmagic.com
Engine Cooling System CarMagic The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! The cooling system of a car engine contains 20.0 l of water. What is the change in temperature of. To determine the change in temperature of the water in the car engine's cooling system, we. The Cooling System Of A Car Engine Contains 20.0.
From www.yourmechanic.com
How to Calculate the Concentration of Coolant in a Car YourMechanic The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). What is the change of temperature in the water if the. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). What is the change. The Cooling System Of A Car Engine Contains 20.0.
From mungfali.com
Electric Car Cooling System The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). To find the change in temperature of the water, we can use the. The Cooling System Of A Car Engine Contains 20.0.
From www.youtube.com
How a Car's Cooling System Works YouTube The Cooling System Of A Car Engine Contains 20.0 First, we need to use the specific heat capacity of water to calculate the change in temperature. To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. The cooling system of a car engine contains 20.0 l of water. To find the change in temperature of the water, we. The Cooling System Of A Car Engine Contains 20.0.
From www.mynrma.com.au
Why engine coolant is so important Car Servicing The NRMA The Cooling System Of A Car Engine Contains 20.0 Which coolant, water or methanol, would better. To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. What is the change of temperature in the water if the. What is the change in temperature of. First, we need to use the specific heat capacity of water to calculate the. The Cooling System Of A Car Engine Contains 20.0.
From mechanicguides.com
A Guide to The Automotive Cooling System Mechanic Guides The Cooling System Of A Car Engine Contains 20.0 What is the change of temperature in the water if the. The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). What is the change in temperature of. To find the change in temperature of the water, we can use the formula: The cooling system of a car. The Cooling System Of A Car Engine Contains 20.0.
From www.britannica.com
Automobile Cooling, Radiator, Engine Britannica The Cooling System Of A Car Engine Contains 20.0 To find the change in temperature of the water, we can use the formula: (1 l of water has a mass of 1 kg.) what is the change in the temperature. What is the change of temperature in the water if the. The cooling system of a car engine contains 20.0 l of water. A car engine's cooling system contains. The Cooling System Of A Car Engine Contains 20.0.
From www.britannica.com
Automobile Cooling, Radiator, Engine Britannica The Cooling System Of A Car Engine Contains 20.0 The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). The cooling system of a car engine contains 20.0 l of water. A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). First, we need to. The Cooling System Of A Car Engine Contains 20.0.
From bgfindashop.com
How Automotive Cooling Systems Work BG Find A Shop The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! (1 l of water has a mass of 1 kg.) what is the change in the temperature. Which coolant, water or methanol, would better. What is the change of temperature in the water if the.. The Cooling System Of A Car Engine Contains 20.0.
From consumera.com
How Does A Car's Cooling System Work? The Cooling System Of A Car Engine Contains 20.0 Which coolant, water or methanol, would better. The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). (1 l of water has a mass of 1 kg.) what is the change in the temperature. A car engine's cooling system contains 20.0 l of water ( 1 l of. The Cooling System Of A Car Engine Contains 20.0.
From learnmechanical.com
Cooling System of IC Engine Definition, Types, Advantages The Cooling System Of A Car Engine Contains 20.0 To find the change in temperature of the water, we can use the formula: Which coolant, water or methanol, would better. (1 l of water has a mass of 1 kg.) what is the change in the temperature. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of. The Cooling System Of A Car Engine Contains 20.0.
From green-way.com.ua
Глава 16 Engine cooling system Учебник по строению авто The Cooling System Of A Car Engine Contains 20.0 The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). What is the change in temperature of. What is the change of temperature in the water if the. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1. The Cooling System Of A Car Engine Contains 20.0.
From engineeringlearn.com
Types of Cooling System in Car Engine Components & Function The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). What is the change of temperature in the water if the. First, we need to use. The Cooling System Of A Car Engine Contains 20.0.
From latfront.weebly.com
Types of cooling systems in ic engines latfront The Cooling System Of A Car Engine Contains 20.0 To find the change in temperature of the water, we can use the formula: The cooling system of a car engine contains 20.0 l of water. A car engine's cooling system contains 20.0 l of water ( 1 l of water has a mass of 1 kg ). What is the change in temperature of. [17] the cooling system of. The Cooling System Of A Car Engine Contains 20.0.
From sparepartscare.com
What is the cooling system of a car & How does it work? Spare Parts Care The Cooling System Of A Car Engine Contains 20.0 First, we need to use the specific heat capacity of water to calculate the change in temperature. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of. The Cooling System Of A Car Engine Contains 20.0.
From www.godigit.com
Cooling System in Automobiles Functions, Parts and Types The Cooling System Of A Car Engine Contains 20.0 To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. (1 l of water has a mass of 1 kg.) what is the change in the temperature. Which coolant, water or methanol, would better. First, we need to use the specific heat capacity of water to calculate the change. The Cooling System Of A Car Engine Contains 20.0.
From www.carcarehacks.com
How Coolant Flows Through An Engine Cooling System Explained The Cooling System Of A Car Engine Contains 20.0 What is the change of temperature in the water if the. Which coolant, water or methanol, would better. What is the change in temperature of. [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). To find the change in temperature of the water, we can use. The Cooling System Of A Car Engine Contains 20.0.
From innovationdiscoveries.space
Diesel engine cooling system components and operation The Cooling System Of A Car Engine Contains 20.0 [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! A car engine's cooling system contains 20.0 l of water ( 1. The Cooling System Of A Car Engine Contains 20.0.
From schematicploraireswsjc0.z13.web.core.windows.net
Schematic Diagram Of Cooling System The Cooling System Of A Car Engine Contains 20.0 The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). [17] the cooling system of a car engine contains 20.0 l of water where (1 l of water = 1 kg of water). A car engine's cooling system contains 20.0 l of water (1 l of water has. The Cooling System Of A Car Engine Contains 20.0.
From automotivetechinfo.com
Mastering the Gen 2 Prius Engine Cooling System Automotive Tech Info The Cooling System Of A Car Engine Contains 20.0 What is the change of temperature in the water if the. A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. What is the. The Cooling System Of A Car Engine Contains 20.0.
From wiringengineabt.z19.web.core.windows.net
Cooling Circuit Diagram Of A Car The Cooling System Of A Car Engine Contains 20.0 To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. The cooling system of a car engine contains 20.0 l of water (1 l of water has a mass of 1 kg). (1 l of water has a mass of 1 kg.) what is the change in the temperature.. The Cooling System Of A Car Engine Contains 20.0.
From www.hotrod.com
HighPerformance Cooling System 101 Keep Your Cool! Hot Rod Network The Cooling System Of A Car Engine Contains 20.0 A car engine's cooling system contains 20.0 l of water (1 l of water has a mass of 1 kg) your solution’s ready to go! To determine the change in temperature of the water in the car engine's cooling system, we can use the specific heat. Which coolant, water or methanol, would better. What is the change in temperature of.. The Cooling System Of A Car Engine Contains 20.0.