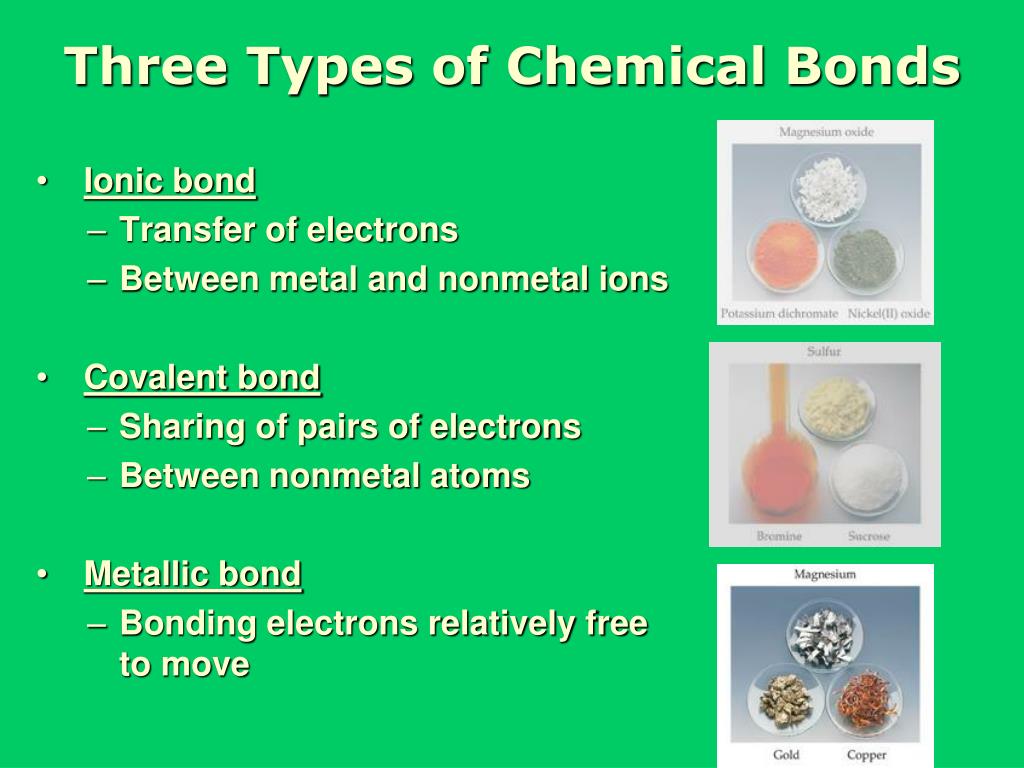
What Are The Three Basic Types Of Chemical Bonds . Learn the definition of a chemical bond. An ionic bond is formed when one atom. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Main types of chemical bonds. A bond between two atoms depends upon the electronegativity difference. Discover the three types of chemical bonds, see examples of each, and understand when the different. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. Different types of bonds form different. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. A chemical bond is the force that holds atoms together in a chemical compound. There are three idealized types of bonding: The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2].
from www.slideserve.com
Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. There are three idealized types of bonding: Learn the definition of a chemical bond. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Discover the three types of chemical bonds, see examples of each, and understand when the different. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Main types of chemical bonds. A chemical bond is the force that holds atoms together in a chemical compound. A bond between two atoms depends upon the electronegativity difference.
PPT Mastering Chemistry PowerPoint Presentation, free download ID
What Are The Three Basic Types Of Chemical Bonds Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. A chemical bond is the force that holds atoms together in a chemical compound. Learn the definition of a chemical bond. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Discover the three types of chemical bonds, see examples of each, and understand when the different. An ionic bond is formed when one atom. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. A bond between two atoms depends upon the electronegativity difference. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. There are three idealized types of bonding: The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Different types of bonds form different. Main types of chemical bonds.
From www.youtube.com
The Different Types of Chemical Bonding (Animation) YouTube What Are The Three Basic Types Of Chemical Bonds Different types of bonds form different. A bond between two atoms depends upon the electronegativity difference. There are three idealized types of bonding: Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Discover the three types of chemical bonds, see examples of each,. What Are The Three Basic Types Of Chemical Bonds.
From www.slideshare.net
The 3 Types of Chemical Bonds by Paolo Agcaoili What Are The Three Basic Types Of Chemical Bonds Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. Main types of chemical bonds. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT CHEMICAL BONDS PowerPoint Presentation, free download ID6445592 What Are The Three Basic Types Of Chemical Bonds Learn the definition of a chemical bond. There are three idealized types of bonding: A chemical bond is the force that holds atoms together in a chemical compound. A bond between two atoms depends upon the electronegativity difference. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. An ionic bond is formed when one. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Unit 7 Bonding & Molecular Geometry PowerPoint Presentation What Are The Three Basic Types Of Chemical Bonds Learn the definition of a chemical bond. Discover the three types of chemical bonds, see examples of each, and understand when the different. Main types of chemical bonds. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. There are three idealized types of bonding: The main types of chemical bonds are ionic bond, covalent. What Are The Three Basic Types Of Chemical Bonds.
From www.slideshare.net
The 3 Types of Chemical Bonds by Paolo Agcaoili What Are The Three Basic Types Of Chemical Bonds The two main types of bonds formed between atoms are ionic bonds and covalent bonds. A chemical bond is the force that holds atoms together in a chemical compound. A bond between two atoms depends upon the electronegativity difference. Different types of bonds form different. Main types of chemical bonds. Discover the three types of chemical bonds, see examples of. What Are The Three Basic Types Of Chemical Bonds.
From sciencenotes.org
Types of Chemical Bonds What Are The Three Basic Types Of Chemical Bonds Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. A chemical bond is the force that holds atoms together in a chemical compound. Main types of chemical bonds. An ionic bond is formed when one atom. Chemical bonds form when electrons can be simultaneously close to two or more. What Are The Three Basic Types Of Chemical Bonds.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples What Are The Three Basic Types Of Chemical Bonds Different types of bonds form different. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Learn the definition of a chemical bond. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. An ionic bond is. What Are The Three Basic Types Of Chemical Bonds.
From www.biyanicolleges.org
types of chemical bonds in chemistry Biyani Group of colleges What Are The Three Basic Types Of Chemical Bonds The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. A chemical bond is the force that holds atoms together in a chemical compound. Learn the definition. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Basic Chemistry PowerPoint Presentation ID543152 What Are The Three Basic Types Of Chemical Bonds Learn the definition of a chemical bond. Main types of chemical bonds. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Discover the three types of chemical bonds, see examples of each, and understand when the different. Chemical bonds are formed when electrons. What Are The Three Basic Types Of Chemical Bonds.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Are The Three Basic Types Of Chemical Bonds Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. There are three idealized types of bonding: Different types of bonds form different. Chemical bonds form when electrons can be simultaneously close to. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Types of chemical bonds PowerPoint Presentation, free download What Are The Three Basic Types Of Chemical Bonds The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Discover the three types of chemical bonds, see examples of each, and understand when the different. Different types of bonds form different. A chemical bond is the force that holds atoms together in a chemical compound. Learn the definition of a chemical bond.. What Are The Three Basic Types Of Chemical Bonds.
From www.colourbox.com
Types of chemical bonding Stock vector Colourbox What Are The Three Basic Types Of Chemical Bonds Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. An ionic bond is formed when one atom. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Chemical bonds are formed when electrons in different atoms interact with. What Are The Three Basic Types Of Chemical Bonds.
From www.slideshare.net
The 3 Types of Chemical Bonds by Paolo Agcaoili What Are The Three Basic Types Of Chemical Bonds An ionic bond is formed when one atom. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Different types of bonds form different. Learn the definition of a chemical bond. A chemical bond is. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Unit 14 Chemical Bonding PowerPoint Presentation, free download What Are The Three Basic Types Of Chemical Bonds Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Main types of chemical bonds. The two main types of bonds formed between atoms are ionic bonds. What Are The Three Basic Types Of Chemical Bonds.
From www.totalassignment.com
What are the Different Types of Chemical Bonds? Total assignment Help What Are The Three Basic Types Of Chemical Bonds Learn the definition of a chemical bond. An ionic bond is formed when one atom. Main types of chemical bonds. A bond between two atoms depends upon the electronegativity difference. A chemical bond is the force that holds atoms together in a chemical compound. Discover the three types of chemical bonds, see examples of each, and understand when the different.. What Are The Three Basic Types Of Chemical Bonds.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Are The Three Basic Types Of Chemical Bonds A chemical bond is the force that holds atoms together in a chemical compound. Different types of bonds form different. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Main types of chemical bonds. There are three idealized types of bonding: Learn the. What Are The Three Basic Types Of Chemical Bonds.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica What Are The Three Basic Types Of Chemical Bonds Main types of chemical bonds. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. A chemical bond is the force that holds atoms together in a chemical compound. There are three idealized types of bonding: Learn the definition of a chemical bond. Chemical bonds are formed when electrons in different atoms interact. What Are The Three Basic Types Of Chemical Bonds.
From learningschooljakobi97.z14.web.core.windows.net
Types Of Chemical Bonds Explained What Are The Three Basic Types Of Chemical Bonds Different types of bonds form different. An ionic bond is formed when one atom. A bond between two atoms depends upon the electronegativity difference. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. Discover. What Are The Three Basic Types Of Chemical Bonds.
From mungfali.com
Chemical Bond Types What Are The Three Basic Types Of Chemical Bonds An ionic bond is formed when one atom. A bond between two atoms depends upon the electronegativity difference. Discover the three types of chemical bonds, see examples of each, and understand when the different. Learn the definition of a chemical bond. A chemical bond is the force that holds atoms together in a chemical compound. The two main types of. What Are The Three Basic Types Of Chemical Bonds.
From studylib.net
Types of Chemical Bonds What Are The Three Basic Types Of Chemical Bonds A bond between two atoms depends upon the electronegativity difference. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. A chemical bond is the force that holds atoms together in a chemical compound. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond. What Are The Three Basic Types Of Chemical Bonds.
From www.youtube.com
Types of Bonding (Ionic, Covalent, Metallic) GCSE Chemistry Revision What Are The Three Basic Types Of Chemical Bonds An ionic bond is formed when one atom. Main types of chemical bonds. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. A chemical bond is the force that holds atoms together in a chemical compound. Chemical bonds are formed when electrons in. What Are The Three Basic Types Of Chemical Bonds.
From www.biorender.com
Types of Chemical Bonds BioRender Science Templates What Are The Three Basic Types Of Chemical Bonds Main types of chemical bonds. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. Learn the definition of a chemical bond. An ionic bond is formed when one atom. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. A chemical bond. What Are The Three Basic Types Of Chemical Bonds.
From www.youtube.com
Chemistry Lesson 31 Different Types of Chemical Bonds YouTube What Are The Three Basic Types Of Chemical Bonds Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. Different types of bonds form different. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. A chemical bond is the force that holds atoms together in a chemical compound. The two main. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint What Are The Three Basic Types Of Chemical Bonds Different types of bonds form different. A chemical bond is the force that holds atoms together in a chemical compound. A bond between two atoms depends upon the electronegativity difference. An ionic bond is formed when one atom. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily. What Are The Three Basic Types Of Chemical Bonds.
From www.chemistrylearner.com
Chemical Bonds Definition, Types, and Examples What Are The Three Basic Types Of Chemical Bonds Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. Discover the three types of chemical bonds, see examples of each, and understand when the different. Main types of chemical bonds. There are three idealized types of bonding: Chemical bonds form when electrons can be simultaneously close to two or. What Are The Three Basic Types Of Chemical Bonds.
From printablezonekling.z19.web.core.windows.net
Types Of Chemical Bonds Explained What Are The Three Basic Types Of Chemical Bonds Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Different types of bonds form different. There are three idealized types of bonding: Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. The. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Types of chemical bonds PowerPoint Presentation, free download What Are The Three Basic Types Of Chemical Bonds An ionic bond is formed when one atom. Learn the definition of a chemical bond. There are three idealized types of bonding: The two main types of bonds formed between atoms are ionic bonds and covalent bonds. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Different types of bonds form different.. What Are The Three Basic Types Of Chemical Bonds.
From mavink.com
Covalent Bond Types What Are The Three Basic Types Of Chemical Bonds A bond between two atoms depends upon the electronegativity difference. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. An ionic bond is formed when one atom. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Discover. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Types of Primary Chemical Bonds PowerPoint Presentation, free What Are The Three Basic Types Of Chemical Bonds Discover the three types of chemical bonds, see examples of each, and understand when the different. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. A chemical bond is the force that holds atoms together in a chemical compound. Learn the definition of. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Noble Gases and Valence e Ionization Energy and Bonding What Are The Three Basic Types Of Chemical Bonds Different types of bonds form different. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. An ionic bond is formed when one atom. Learn the definition of a chemical bond. Main types. What Are The Three Basic Types Of Chemical Bonds.
From www.slideshare.net
10/26 What are the 3 types of chemical bonds? Part II What Are The Three Basic Types Of Chemical Bonds Different types of bonds form different. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. An ionic bond is formed when one atom.. What Are The Three Basic Types Of Chemical Bonds.
From www.shutterstock.com
Chemical Bonds Infographic Diagram Showing Types Stock Illustration What Are The Three Basic Types Of Chemical Bonds Learn the definition of a chemical bond. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. An ionic bond is formed when one atom. A chemical bond is the force that holds atoms together in a chemical compound. There are three idealized types of bonding: Chemical bonds are formed when electrons in. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Mastering Chemistry PowerPoint Presentation, free download ID What Are The Three Basic Types Of Chemical Bonds A chemical bond is the force that holds atoms together in a chemical compound. There are three idealized types of bonding: Different types of bonds form different. Learn the definition of a chemical bond. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more. The main types of chemical bonds. What Are The Three Basic Types Of Chemical Bonds.
From www.slideserve.com
PPT Levels of Organization in the Human Body PowerPoint Presentation What Are The Three Basic Types Of Chemical Bonds Different types of bonds form different. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. A bond between two atoms depends upon the electronegativity difference. A chemical bond is the force that holds atoms together in a chemical compound. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but. What Are The Three Basic Types Of Chemical Bonds.
From www.phdnest.com
Covalent Bond Meaning, Types, Properties, Examples PhD Nest What Are The Three Basic Types Of Chemical Bonds The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Learn the definition of a chemical bond. Different types of bonds form different. Discover the three types of chemical bonds, see examples of each, and understand when the. What Are The Three Basic Types Of Chemical Bonds.