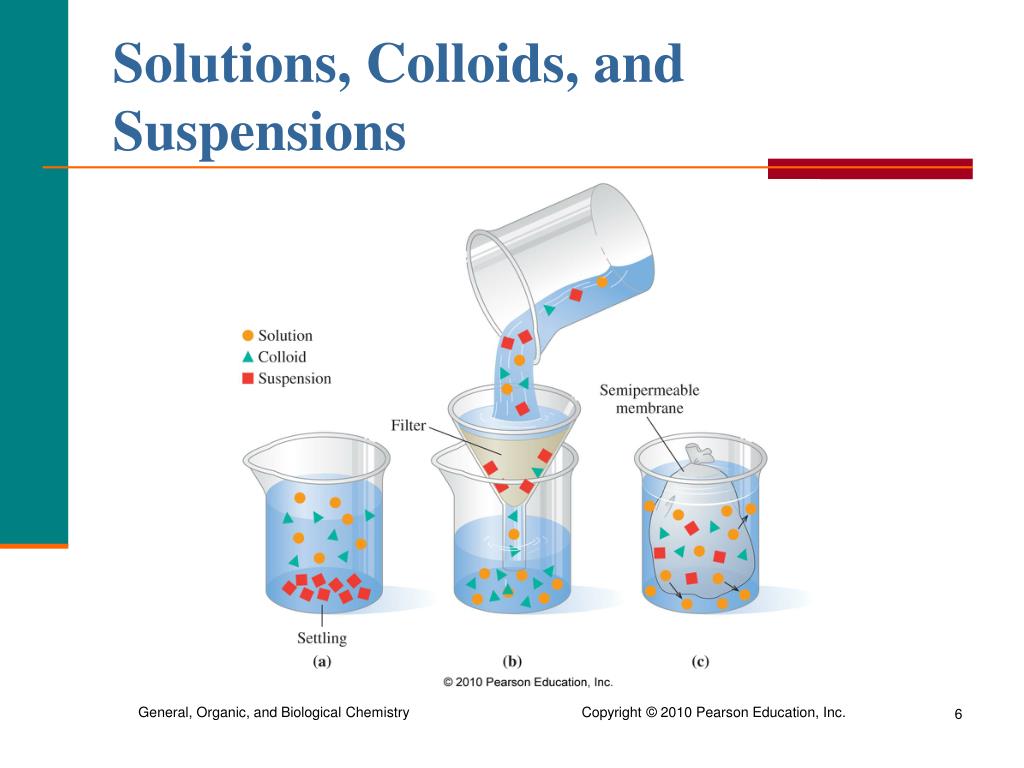
Differentiate Between Solution Suspension And Colloid With Examples . Hydrophilic colloids contain an outer shell of groups that interact favorably. The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. The particles in a solution are. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. In this animated lecture, i will teach you about solution, suspension, colloid and. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect.
from www.slideserve.com
A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. The particles in a solution are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. In this animated lecture, i will teach you about solution, suspension, colloid and. The main difference between colloid and suspension lies in the size of particles. Hydrophilic colloids contain an outer shell of groups that interact favorably. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloid particles are much smaller than suspension particles.
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID
Differentiate Between Solution Suspension And Colloid With Examples A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The particles in a solution are. Hydrophilic colloids contain an outer shell of groups that interact favorably. In this animated lecture, i will teach you about solution, suspension, colloid and. Colloid particles are much smaller than suspension particles. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Differentiate Between Solution Suspension And Colloid With Examples A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloid particles are much smaller than suspension particles. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. The main difference between colloid and suspension lies in the. Differentiate Between Solution Suspension And Colloid With Examples.
From scsscience9.blogspot.com
Is Matter Around Us Pure? Blog 6 Differentiate Between Solution Suspension And Colloid With Examples A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. The particles in a solution are. In this animated lecture, i will teach you about solution, suspension, colloid and. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of. Differentiate Between Solution Suspension And Colloid With Examples.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Differentiate Between Solution Suspension And Colloid With Examples Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Hydrophilic colloids contain an outer shell of groups that interact favorably. Colloid particles are much smaller than suspension particles.. Differentiate Between Solution Suspension And Colloid With Examples.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Differentiate Between Solution Suspension And Colloid With Examples The main difference between colloid and suspension lies in the size of particles. Hydrophilic colloids contain an outer shell of groups that interact favorably. The particles in a solution are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Colloid particles are much smaller than suspension particles. A colloid. Differentiate Between Solution Suspension And Colloid With Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Differentiate Between Solution Suspension And Colloid With Examples A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Colloid particles are much smaller than suspension particles. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. In this animated lecture, i will teach you about. Differentiate Between Solution Suspension And Colloid With Examples.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Differentiate Between Solution Suspension And Colloid With Examples Colloid particles are much smaller than suspension particles. In this animated lecture, i will teach you about solution, suspension, colloid and. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one. Differentiate Between Solution Suspension And Colloid With Examples.
From www.studypool.com
SOLUTION Difference between solution colloids and suspension. Studypool Differentiate Between Solution Suspension And Colloid With Examples The particles in a solution are. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The main difference between colloid and suspension lies in the size of particles. Hydrophilic colloids contain an. Differentiate Between Solution Suspension And Colloid With Examples.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Differentiate Between Solution Suspension And Colloid With Examples In this animated lecture, i will teach you about solution, suspension, colloid and. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. The particles in a solution are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The main difference. Differentiate Between Solution Suspension And Colloid With Examples.
From www.toppr.com
(k) Differentiate between solution, suspension and colloids on the Differentiate Between Solution Suspension And Colloid With Examples A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Hydrophilic colloids contain an outer shell of groups that interact favorably. Colloid particles are much smaller than suspension particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Suspensions and. Differentiate Between Solution Suspension And Colloid With Examples.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Differentiate Between Solution Suspension And Colloid With Examples The main difference between colloid and suspension lies in the size of particles. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The particles in a solution are. A colloid can. Differentiate Between Solution Suspension And Colloid With Examples.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Differentiate Between Solution Suspension And Colloid With Examples In this animated lecture, i will teach you about solution, suspension, colloid and. Hydrophilic colloids contain an outer shell of groups that interact favorably. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture. Differentiate Between Solution Suspension And Colloid With Examples.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Differentiate Between Solution Suspension And Colloid With Examples The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. Hydrophilic colloids contain an outer shell of groups that interact favorably. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A colloid can be distinguished from a true. Differentiate Between Solution Suspension And Colloid With Examples.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Differentiate Between Solution Suspension And Colloid With Examples A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Colloid particles are much smaller than suspension particles. The particles in a solution are. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In this animated lecture,. Differentiate Between Solution Suspension And Colloid With Examples.
From www.youtube.com
Solution, Suspension and Colloid aumsum kids education science Differentiate Between Solution Suspension And Colloid With Examples A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The particles in a solution are. In this animated lecture, i will teach you about solution, suspension,. Differentiate Between Solution Suspension And Colloid With Examples.
From brainly.in
what is the different between solution suspension and colloid Brainly.in Differentiate Between Solution Suspension And Colloid With Examples A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In this animated lecture, i will teach you about solution, suspension, colloid and. The particles in a solution are. Colloid particles are much smaller than suspension particles. Hydrophilic colloids contain an outer shell of groups that interact favorably. Suspensions and colloids. Differentiate Between Solution Suspension And Colloid With Examples.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Differentiate Between Solution Suspension And Colloid With Examples Hydrophilic colloids contain an outer shell of groups that interact favorably. Colloid particles are much smaller than suspension particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The main difference between colloid and suspension lies in the size of particles. Suspensions and colloids differ from solution in that a. Differentiate Between Solution Suspension And Colloid With Examples.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Differentiate Between Solution Suspension And Colloid With Examples The particles in a solution are. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. The main difference between colloid and suspension lies in the size of particles.. Differentiate Between Solution Suspension And Colloid With Examples.
From www.slideserve.com
PPT Solution and Suspension PowerPoint Presentation, free download Differentiate Between Solution Suspension And Colloid With Examples Hydrophilic colloids contain an outer shell of groups that interact favorably. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Colloid particles are much smaller than suspension particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of. Differentiate Between Solution Suspension And Colloid With Examples.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Differentiate Between Solution Suspension And Colloid With Examples A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The particles in a solution are. The main difference between colloid and suspension lies in the size of particles. Hydrophilic colloids contain an outer shell of groups that interact favorably. Suspensions and colloids differ from solution in that a solution. Differentiate Between Solution Suspension And Colloid With Examples.
From brainly.in
[Solved] Distinguish between true solution , colloid and suspension on Differentiate Between Solution Suspension And Colloid With Examples The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. In this animated lecture, i will teach you about solution, suspension, colloid and. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. The particles in a solution are. A colloid. Differentiate Between Solution Suspension And Colloid With Examples.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Differentiate Between Solution Suspension And Colloid With Examples The particles in a solution are. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Colloid particles are much smaller than suspension particles. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a mixture. Differentiate Between Solution Suspension And Colloid With Examples.
From pediaa.com
Difference Between True Solution and Colloidal Dispersion Definition Differentiate Between Solution Suspension And Colloid With Examples A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. In this animated lecture, i will teach you about solution, suspension, colloid and. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. The particles in a solution are. Hydrophilic colloids contain. Differentiate Between Solution Suspension And Colloid With Examples.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Differentiate Between Solution Suspension And Colloid With Examples In this animated lecture, i will teach you about solution, suspension, colloid and. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A colloid is a. Differentiate Between Solution Suspension And Colloid With Examples.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Differentiate Between Solution Suspension And Colloid With Examples In this animated lecture, i will teach you about solution, suspension, colloid and. The main difference between colloid and suspension lies in the size of particles. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Suspensions and colloids differ from solution in that a solution is. Differentiate Between Solution Suspension And Colloid With Examples.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2182578 Differentiate Between Solution Suspension And Colloid With Examples The particles in a solution are. Colloid particles are much smaller than suspension particles. The main difference between colloid and suspension lies in the size of particles. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size. Differentiate Between Solution Suspension And Colloid With Examples.
From studylib.net
Solutions, Colloids, Suspension Differentiate Between Solution Suspension And Colloid With Examples The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a heterogeneous mixture in which the. Differentiate Between Solution Suspension And Colloid With Examples.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Differentiate Between Solution Suspension And Colloid With Examples A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The main difference between colloid and suspension lies in the size of particles. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Colloid particles are much smaller than suspension particles. The. Differentiate Between Solution Suspension And Colloid With Examples.
From brainly.in
draw diagram illustrate true solutions suspension and colloidal Differentiate Between Solution Suspension And Colloid With Examples A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The particles in a solution are. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloid can be distinguished from a true solution by its ability to scatter a. Differentiate Between Solution Suspension And Colloid With Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Differentiate Between Solution Suspension And Colloid With Examples Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Colloid particles are much smaller than suspension particles. Hydrophilic colloids contain an outer shell of groups that interact favorably.. Differentiate Between Solution Suspension And Colloid With Examples.
From www.differencebetween.net
Difference Between Colloid and Suspension Difference Between Differentiate Between Solution Suspension And Colloid With Examples The particles in a solution are. The main difference between colloid and suspension lies in the size of particles. Hydrophilic colloids contain an outer shell of groups that interact favorably. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloid is a mixture in which one of the soluble. Differentiate Between Solution Suspension And Colloid With Examples.
From www.pinterest.com
mixture suspension colloid Google Search Chemistry worksheets Differentiate Between Solution Suspension And Colloid With Examples A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. In this animated lecture, i will teach you about solution, suspension, colloid and. Hydrophilic colloids contain an outer shell of groups that interact. Differentiate Between Solution Suspension And Colloid With Examples.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in Differentiate Between Solution Suspension And Colloid With Examples A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is. Differentiate Between Solution Suspension And Colloid With Examples.
From edurev.in
Difference between true solution, suspension and colloidal sol Differentiate Between Solution Suspension And Colloid With Examples Hydrophilic colloids contain an outer shell of groups that interact favorably. In this animated lecture, i will teach you about solution, suspension, colloid and. The particles in a solution are. The main difference between colloid and suspension lies in the size of particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those. Differentiate Between Solution Suspension And Colloid With Examples.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Differentiate Between Solution Suspension And Colloid With Examples Hydrophilic colloids contain an outer shell of groups that interact favorably. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The main difference between colloid and suspension lies in the size of particles. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and. Differentiate Between Solution Suspension And Colloid With Examples.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Differentiate Between Solution Suspension And Colloid With Examples The main difference between colloid and suspension lies in the size of particles. The particles in a solution are. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Hydrophilic colloids contain. Differentiate Between Solution Suspension And Colloid With Examples.