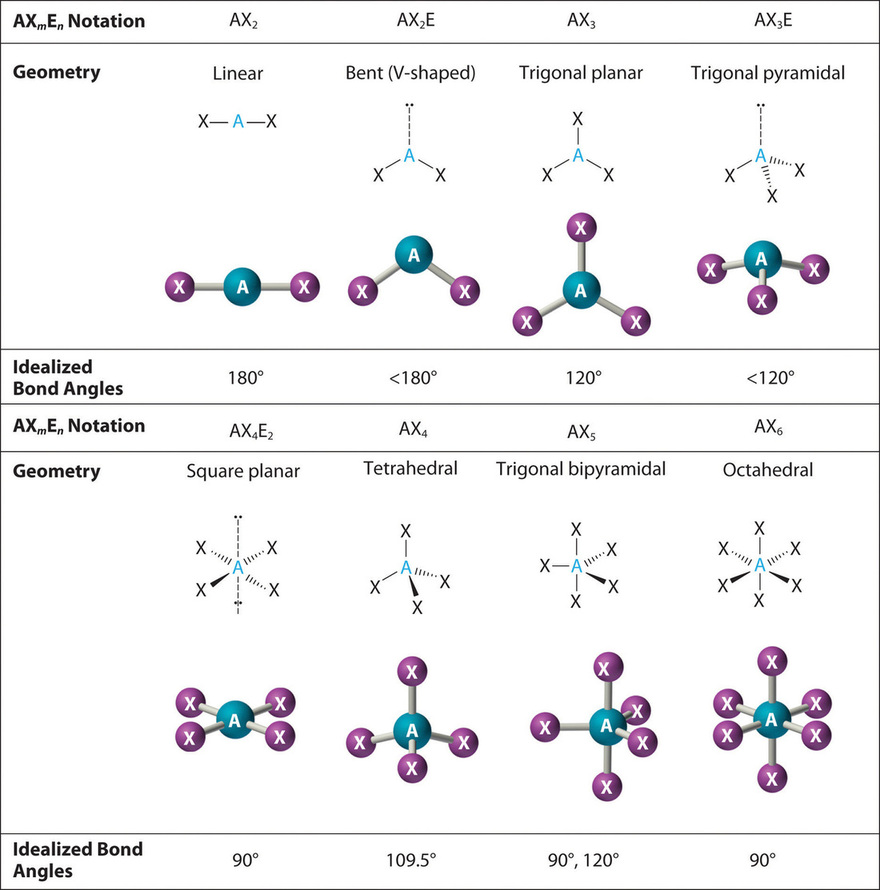
How Does The Vsepr Theory Explain Molecular Shape . the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. this shape is called bent or angular. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. A molecule with four electron groups about the central atom orients the four groups in the.
from chem.libretexts.org
this shape is called bent or angular. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. A molecule with four electron groups about the central atom orients the four groups in the. vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry.
9.2 The VSEPR Model Chemistry LibreTexts
How Does The Vsepr Theory Explain Molecular Shape vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. A molecule with four electron groups about the central atom orients the four groups in the. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. this shape is called bent or angular.
From www.chemistrylearner.com
VSEPR Theory Explanation, Chart, and Examples How Does The Vsepr Theory Explain Molecular Shape valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and. How Does The Vsepr Theory Explain Molecular Shape.
From jsmithmoore.com
Vsepr theory chart How Does The Vsepr Theory Explain Molecular Shape A molecule with four electron groups about the central atom orients the four groups in the. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. vsepr theory is used to. How Does The Vsepr Theory Explain Molecular Shape.
From www.studypool.com
SOLUTION Vsepr theory molecular shapes chart Studypool How Does The Vsepr Theory Explain Molecular Shape the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. this shape is called bent or angular. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. A molecule with four. How Does The Vsepr Theory Explain Molecular Shape.
From jsmithmoore.com
Vsepr theory chart How Does The Vsepr Theory Explain Molecular Shape the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. the valence shell electron pair repulsion (vsepr) theory is a simple and useful. How Does The Vsepr Theory Explain Molecular Shape.
From jsmithmoore.com
Vsepr theory chart How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. this shape is called bent or angular. the vsepr theory assumes that. How Does The Vsepr Theory Explain Molecular Shape.
From brunofuga.adv.br
VSEPR Theory Explanation, Chart, And Examples, 51 OFF How Does The Vsepr Theory Explain Molecular Shape valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. A molecule with four electron groups about the central atom orients the four groups in the. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. the vsepr theory assumes that. How Does The Vsepr Theory Explain Molecular Shape.
From www.vrogue.co
Molecular Shapes Vsepr Theory vrogue.co How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. valence shell electron pair repulsion or vsepr theory can be used to predict. How Does The Vsepr Theory Explain Molecular Shape.
From www.chemistrysteps.com
VSEPR Theory Geometry of Organic Molecules Chemistry Steps How Does The Vsepr Theory Explain Molecular Shape the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. this shape is called bent or angular. vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. the theory is based on lewis structures. How Does The Vsepr Theory Explain Molecular Shape.
From printablelistmcguire.z22.web.core.windows.net
Ap Chemistry Molecular Geometry Worksheets How Does The Vsepr Theory Explain Molecular Shape this shape is called bent or angular. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. the vsepr theory assumes that each atom in a molecule will achieve a. How Does The Vsepr Theory Explain Molecular Shape.
From www.pinterest.com.au
Pin on Chemistry Basic Chemistry How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. valence shell electron pair repulsion or vsepr theory can be used. How Does The Vsepr Theory Explain Molecular Shape.
From chem.libretexts.org
1.2 VSEPR Theory The Five Basic Shapes Chemistry LibreTexts How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. A molecule with four electron groups about the central atom orients the. How Does The Vsepr Theory Explain Molecular Shape.
From quizizz.com
VSEPR Theory Chemistry Quiz Quizizz How Does The Vsepr Theory Explain Molecular Shape the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. vsepr theory is used to predict the arrangement of electron pairs around central. How Does The Vsepr Theory Explain Molecular Shape.
From jsmithmoore.com
Vsepr theory chart How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. A molecule with four electron groups about the central atom orients the four groups in the. vsepr theory is. How Does The Vsepr Theory Explain Molecular Shape.
From mungfali.com
VSEPR Theory Table How Does The Vsepr Theory Explain Molecular Shape valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. this shape is called bent or angular. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the theory is based on lewis structures and the simple idea. How Does The Vsepr Theory Explain Molecular Shape.
From www.teacharesources.com
Grade 11 Shapes of molecules and the VSEPR model for shapes in How Does The Vsepr Theory Explain Molecular Shape vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion (vsepr) theory is a simple and useful. How Does The Vsepr Theory Explain Molecular Shape.
From chemsjc.blogspot.com
Jimchem VSEPR Theory How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. A molecule with four electron groups about the central atom orients the four groups in the. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. this shape is. How Does The Vsepr Theory Explain Molecular Shape.
From www.vrogue.co
Includegraphics Scale1 0 Vsepr Shapes Eps Chemistry S vrogue.co How Does The Vsepr Theory Explain Molecular Shape this shape is called bent or angular. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the theory is based on. How Does The Vsepr Theory Explain Molecular Shape.
From www.w3schools.blog
VSEPR Theory W3schools How Does The Vsepr Theory Explain Molecular Shape A molecule with four electron groups about the central atom orients the four groups in the. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central. How Does The Vsepr Theory Explain Molecular Shape.
From chem.libretexts.org
9.2 The VSEPR Model Chemistry LibreTexts How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. the vsepr theory assumes that each atom in a molecule will achieve a. How Does The Vsepr Theory Explain Molecular Shape.
From study.com
VSEPR Theory Chart & Model Lesson How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. valence shell electron pair repulsion or vsepr theory can be used. How Does The Vsepr Theory Explain Molecular Shape.
From wisc.pb.unizin.org
Day 11 Resonance Structures, VSEPR Theory Chemistry 109 How Does The Vsepr Theory Explain Molecular Shape A molecule with four electron groups about the central atom orients the four groups in the. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. this shape is called bent or angular. vsepr theory is used to predict the arrangement of electron pairs. How Does The Vsepr Theory Explain Molecular Shape.
From mavink.com
Vsepr Theory Shapes How Does The Vsepr Theory Explain Molecular Shape A molecule with four electron groups about the central atom orients the four groups in the. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central. How Does The Vsepr Theory Explain Molecular Shape.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules How Does The Vsepr Theory Explain Molecular Shape the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. this shape is called bent or angular. A molecule with four electron groups. How Does The Vsepr Theory Explain Molecular Shape.
From www.numerade.com
SOLVEDUse the VSEPR theory to predict the shape of (a) the molecule How Does The Vsepr Theory Explain Molecular Shape vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. this shape is called bent or angular. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. A molecule with four electron groups. How Does The Vsepr Theory Explain Molecular Shape.
From mavink.com
Vsepr Molecular Shapes Chart How Does The Vsepr Theory Explain Molecular Shape the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. valence shell electron pair repulsion or vsepr theory can be used to predict. How Does The Vsepr Theory Explain Molecular Shape.
From www.pinterest.com
VSEPR theory and molecular shapes Molecular geometry, Science How Does The Vsepr Theory Explain Molecular Shape the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one. A molecule with four electron groups about the central atom orients the. How Does The Vsepr Theory Explain Molecular Shape.
From www.sigmaaldrich.com
VSEPR Chart Valence Shell Electron Pair Repulsion Theory How Does The Vsepr Theory Explain Molecular Shape vsepr theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. this shape is called bent or angular. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. A molecule with four electron groups about the central atom orients the four groups. How Does The Vsepr Theory Explain Molecular Shape.
From www.youtube.com
CHEMISTRY 101 Apply VSEPR Theory to predict molecular geometry YouTube How Does The Vsepr Theory Explain Molecular Shape A molecule with four electron groups about the central atom orients the four groups in the. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the. How Does The Vsepr Theory Explain Molecular Shape.
From mavink.com
Vsepr Summary Chart How Does The Vsepr Theory Explain Molecular Shape A molecule with four electron groups about the central atom orients the four groups in the. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the. How Does The Vsepr Theory Explain Molecular Shape.
From www.aakash.ac.in
VSEPR Theory Postulates, Prediction of geometrical shapes, Limitations How Does The Vsepr Theory Explain Molecular Shape this shape is called bent or angular. the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. A molecule with four electron groups about the central atom orients the four groups in the. the vsepr theory assumes that each atom in a molecule will achieve a. How Does The Vsepr Theory Explain Molecular Shape.
From www.expii.com
VSEPR Theory — Definition & Overview Expii How Does The Vsepr Theory Explain Molecular Shape the valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is. How Does The Vsepr Theory Explain Molecular Shape.