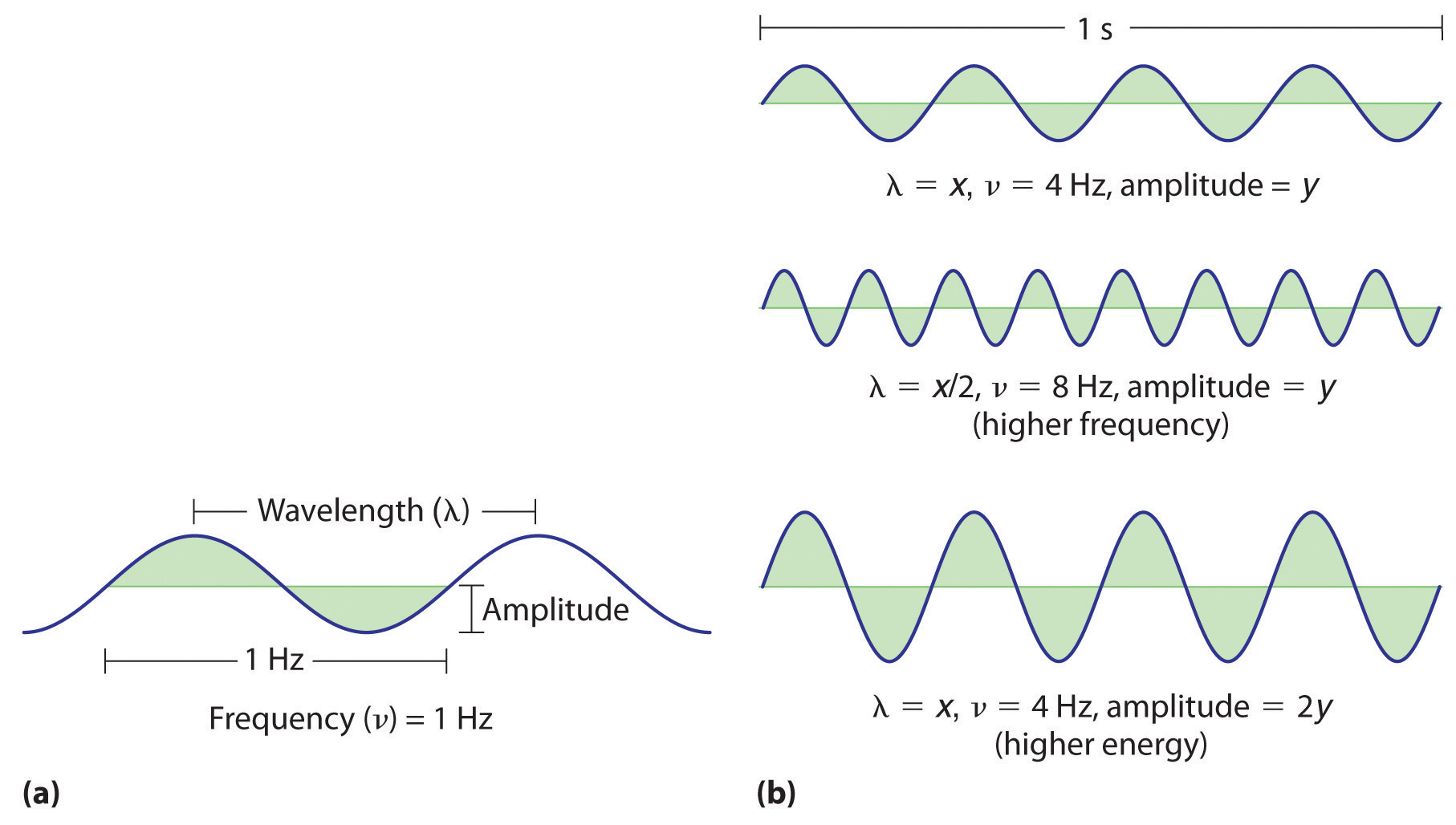
Low Frequency High Energy . The energy is simply the photon’s frequency multiplied by the planck constant (h). Frequency and wavelength are inverse correlated by way of the speed of light (c): If the energy of each wavelength is considered to be a. the relationship between energy (e), frequency and wavelength can be described with this equation: the energy of the wave depends on both the amplitude and the frequency. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. High frequency light has high. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. the amount of energy in a light wave is proportionally related to its frequency: E=hf=\frac {hc} {\lambda} e = hf = λhc.
from chem.libretexts.org
E=hf=\frac {hc} {\lambda} e = hf = λhc. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. High frequency light has high. The energy is simply the photon’s frequency multiplied by the planck constant (h). Frequency and wavelength are inverse correlated by way of the speed of light (c): the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. the amount of energy in a light wave is proportionally related to its frequency: If the energy of each wavelength is considered to be a. the energy of the wave depends on both the amplitude and the frequency. the relationship between energy (e), frequency and wavelength can be described with this equation:
5.1 The Nature of Radiant Energy and the Spectrum
Low Frequency High Energy the energy of the wave depends on both the amplitude and the frequency. the amount of energy in a light wave is proportionally related to its frequency: If the energy of each wavelength is considered to be a. the relationship between energy (e), frequency and wavelength can be described with this equation: E=hf=\frac {hc} {\lambda} e = hf = λhc. High frequency light has high. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. The energy is simply the photon’s frequency multiplied by the planck constant (h). the energy of the wave depends on both the amplitude and the frequency. Frequency and wavelength are inverse correlated by way of the speed of light (c): the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy.
From iotdesignpro.com
An Overview of RF Energy Harvesting Working and Applications Low Frequency High Energy the amount of energy in a light wave is proportionally related to its frequency: Frequency and wavelength are inverse correlated by way of the speed of light (c): High frequency light has high. The energy is simply the photon’s frequency multiplied by the planck constant (h). the energy of the wave depends on both the amplitude and the. Low Frequency High Energy.
From www.numerade.com
SOLVED few different types of radiation are listed in Low Frequency High Energy High frequency light has high. Frequency and wavelength are inverse correlated by way of the speed of light (c): the relationship between energy (e), frequency and wavelength can be described with this equation: the energy of the wave depends on both the amplitude and the frequency. the individual photons have more energy, but this does not mean. Low Frequency High Energy.
From www.chegg.com
Solved A few different types of radiation Low Frequency High Energy E=hf=\frac {hc} {\lambda} e = hf = λhc. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. If the energy of each wavelength is considered to be a. Frequency and wavelength are inverse correlated by way of the speed of light (c): High frequency light has high.. Low Frequency High Energy.
From ar.inspiredpencil.com
High Energy Waves Low Frequency High Energy The energy is simply the photon’s frequency multiplied by the planck constant (h). Frequency and wavelength are inverse correlated by way of the speed of light (c): If the energy of each wavelength is considered to be a. E=hf=\frac {hc} {\lambda} e = hf = λhc. the energy of the wave depends on both the amplitude and the frequency.. Low Frequency High Energy.
From ar.inspiredpencil.com
Spectrum Frequency Wavelength Energy Low Frequency High Energy High frequency light has high. The energy is simply the photon’s frequency multiplied by the planck constant (h). the energy of the wave depends on both the amplitude and the frequency. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. the relationship between energy (e),. Low Frequency High Energy.
From socratic.org
Which type of energy has a shorter wavelength than Low Frequency High Energy The energy is simply the photon’s frequency multiplied by the planck constant (h). the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. High frequency light has high. the amount of energy in a light wave is proportionally related to its frequency: If the energy of each. Low Frequency High Energy.
From www.vectorstock.com
Frequency low and high waves Royalty Free Vector Image Low Frequency High Energy High frequency light has high. The energy is simply the photon’s frequency multiplied by the planck constant (h). the energy of the wave depends on both the amplitude and the frequency. Frequency and wavelength are inverse correlated by way of the speed of light (c): the individual photons have more energy, but this does not mean the total. Low Frequency High Energy.
From www.chegg.com
Solved A few different types of radiation Low Frequency High Energy Frequency and wavelength are inverse correlated by way of the speed of light (c): If the energy of each wavelength is considered to be a. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. High frequency light has high. the relationship between energy (e), frequency and. Low Frequency High Energy.
From www.numerade.com
Use the diagram above to answer the following questions Which wave has Low Frequency High Energy the relationship between energy (e), frequency and wavelength can be described with this equation: the amount of energy in a light wave is proportionally related to its frequency: The energy is simply the photon’s frequency multiplied by the planck constant (h). the individual photons have more energy, but this does not mean the total energy in the. Low Frequency High Energy.
From www.chegg.com
Solved The following is a diagram of energy states and Low Frequency High Energy the energy of the wave depends on both the amplitude and the frequency. the amount of energy in a light wave is proportionally related to its frequency: the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. The energy is simply the photon’s frequency multiplied by. Low Frequency High Energy.
From socratic.org
What color of light has the most energy? Socratic Low Frequency High Energy Frequency and wavelength are inverse correlated by way of the speed of light (c): The energy is simply the photon’s frequency multiplied by the planck constant (h). If the energy of each wavelength is considered to be a. the amount of energy in a light wave is proportionally related to its frequency: High frequency light has high. the. Low Frequency High Energy.
From dxoxtcpnv.blob.core.windows.net
Wavelength Of Microwaves Frequency at Connie blog Low Frequency High Energy If the energy of each wavelength is considered to be a. the energy of the wave depends on both the amplitude and the frequency. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. High frequency light has high. The energy is simply the photon’s frequency multiplied. Low Frequency High Energy.
From www.brettelliott.com
Ultimate Energy Healing Part 5 What is Healing Energy Brett Elliott Low Frequency High Energy E=hf=\frac {hc} {\lambda} e = hf = λhc. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. High frequency light has high. The energy is simply the photon’s frequency multiplied by the planck constant (h). the amount of energy in a light wave is proportionally related. Low Frequency High Energy.
From www.thinglink.com
The Spectrum Low Frequency High Energy High frequency light has high. Frequency and wavelength are inverse correlated by way of the speed of light (c): The energy is simply the photon’s frequency multiplied by the planck constant (h). the amount of energy in a light wave is proportionally related to its frequency: the energy of the wave depends on both the amplitude and the. Low Frequency High Energy.
From www.chegg.com
Solved The following is a diagram of energy states and Low Frequency High Energy If the energy of each wavelength is considered to be a. The energy is simply the photon’s frequency multiplied by the planck constant (h). the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. 13 rows thus low frequency (lf) is also called long wave (lw), medium. Low Frequency High Energy.
From philschatz.com
Energy · Chemistry Low Frequency High Energy the energy of the wave depends on both the amplitude and the frequency. The energy is simply the photon’s frequency multiplied by the planck constant (h). If the energy of each wavelength is considered to be a. the amount of energy in a light wave is proportionally related to its frequency: E=hf=\frac {hc} {\lambda} e = hf =. Low Frequency High Energy.
From chem.libretexts.org
5.1 The Nature of Radiant Energy and the Spectrum Low Frequency High Energy The energy is simply the photon’s frequency multiplied by the planck constant (h). High frequency light has high. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. the amount of energy in a light wave is proportionally related to its frequency: 13 rows thus low. Low Frequency High Energy.
From lakhimi.blogspot.com
PAPER 604 RADIATION (EMR) Low Frequency High Energy High frequency light has high. E=hf=\frac {hc} {\lambda} e = hf = λhc. Frequency and wavelength are inverse correlated by way of the speed of light (c): If the energy of each wavelength is considered to be a. the energy of the wave depends on both the amplitude and the frequency. The energy is simply the photon’s frequency multiplied. Low Frequency High Energy.
From www.faithfulscience.com
Classical Physics Low Frequency High Energy E=hf=\frac {hc} {\lambda} e = hf = λhc. If the energy of each wavelength is considered to be a. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. The energy is simply the photon’s frequency multiplied by the planck constant (h). Frequency and wavelength are inverse correlated. Low Frequency High Energy.
From emc2-igcse-help.blogspot.com
IGCSE Physics 3.11 identify the order of the spectrum Low Frequency High Energy the amount of energy in a light wave is proportionally related to its frequency: the relationship between energy (e), frequency and wavelength can be described with this equation: the energy of the wave depends on both the amplitude and the frequency. E=hf=\frac {hc} {\lambda} e = hf = λhc. If the energy of each wavelength is considered. Low Frequency High Energy.
From telegra.ph
Understanding the Spectrum Telegraph Low Frequency High Energy If the energy of each wavelength is considered to be a. The energy is simply the photon’s frequency multiplied by the planck constant (h). the amount of energy in a light wave is proportionally related to its frequency: High frequency light has high. E=hf=\frac {hc} {\lambda} e = hf = λhc. the energy of the wave depends on. Low Frequency High Energy.
From www.checkinfrared.com
Far Infrared Sauna vs Near or Middle Infrared Low Frequency High Energy the energy of the wave depends on both the amplitude and the frequency. the relationship between energy (e), frequency and wavelength can be described with this equation: High frequency light has high. The energy is simply the photon’s frequency multiplied by the planck constant (h). Frequency and wavelength are inverse correlated by way of the speed of light. Low Frequency High Energy.
From www.slideserve.com
PPT Radiation PowerPoint Presentation, free download Low Frequency High Energy the relationship between energy (e), frequency and wavelength can be described with this equation: 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. the energy of the wave depends on both the amplitude and the frequency. The energy is simply the photon’s frequency multiplied by. Low Frequency High Energy.
From ar.inspiredpencil.com
Frequency Chart Low Frequency High Energy the energy of the wave depends on both the amplitude and the frequency. High frequency light has high. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. the amount of energy in a light wave is proportionally related to its frequency: the relationship between. Low Frequency High Energy.
From www.chegg.com
Solved Place types of radiation listed below Low Frequency High Energy the amount of energy in a light wave is proportionally related to its frequency: The energy is simply the photon’s frequency multiplied by the planck constant (h). If the energy of each wavelength is considered to be a. High frequency light has high. the energy of the wave depends on both the amplitude and the frequency. the. Low Frequency High Energy.
From science8sc.weebly.com
Frequency 8THGRADE SCIENCE Low Frequency High Energy E=hf=\frac {hc} {\lambda} e = hf = λhc. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. If the energy of each wavelength is considered to be a. High frequency light has high. the amount of energy in a light wave is proportionally related to its. Low Frequency High Energy.
From brainly.com
The picture lists light waves from left to right in order of highest to Low Frequency High Energy the relationship between energy (e), frequency and wavelength can be described with this equation: 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. Frequency and wavelength are inverse correlated by way of the speed of light (c): High frequency light has high. E=hf=\frac {hc} {\lambda} e. Low Frequency High Energy.
From courses.lumenlearning.com
The Properties of Light Microbiology Low Frequency High Energy The energy is simply the photon’s frequency multiplied by the planck constant (h). If the energy of each wavelength is considered to be a. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. the individual photons have more energy, but this does not mean the total. Low Frequency High Energy.
From worksheettranciatoog.z21.web.core.windows.net
Printable Emotional Vibrational Frequency Chart Low Frequency High Energy If the energy of each wavelength is considered to be a. Frequency and wavelength are inverse correlated by way of the speed of light (c): the relationship between energy (e), frequency and wavelength can be described with this equation: High frequency light has high. E=hf=\frac {hc} {\lambda} e = hf = λhc. the energy of the wave depends. Low Frequency High Energy.
From jeopardylabs.com
Science 8 Trimester 1 Jeopardy Template Low Frequency High Energy the energy of the wave depends on both the amplitude and the frequency. the relationship between energy (e), frequency and wavelength can be described with this equation: Frequency and wavelength are inverse correlated by way of the speed of light (c): The energy is simply the photon’s frequency multiplied by the planck constant (h). If the energy of. Low Frequency High Energy.
From www.chegg.com
Solved A few different types of radiation Low Frequency High Energy the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. the amount of energy in a light wave is proportionally related to its frequency: The. Low Frequency High Energy.
From www.chegg.com
Solved A few different types of radiation Low Frequency High Energy the amount of energy in a light wave is proportionally related to its frequency: the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. The. Low Frequency High Energy.
From www.researchgate.net
Comparison of wavelength and frequency for the Low Frequency High Energy E=hf=\frac {hc} {\lambda} e = hf = λhc. the individual photons have more energy, but this does not mean the total energy in the wave has a higher energy. High frequency light has high. If the energy of each wavelength is considered to be a. 13 rows thus low frequency (lf) is also called long wave (lw), medium. Low Frequency High Energy.
From www.omnicalculator.com
Frequency Calculator Period to Frequency and More Low Frequency High Energy If the energy of each wavelength is considered to be a. the amount of energy in a light wave is proportionally related to its frequency: the energy of the wave depends on both the amplitude and the frequency. The energy is simply the photon’s frequency multiplied by the planck constant (h). Frequency and wavelength are inverse correlated by. Low Frequency High Energy.
From www.youtube.com
Aleks Understanding the organization of the spectrum Low Frequency High Energy If the energy of each wavelength is considered to be a. the energy of the wave depends on both the amplitude and the frequency. 13 rows thus low frequency (lf) is also called long wave (lw), medium frequency (mf) is also called medium wave (mw),. the relationship between energy (e), frequency and wavelength can be described with. Low Frequency High Energy.