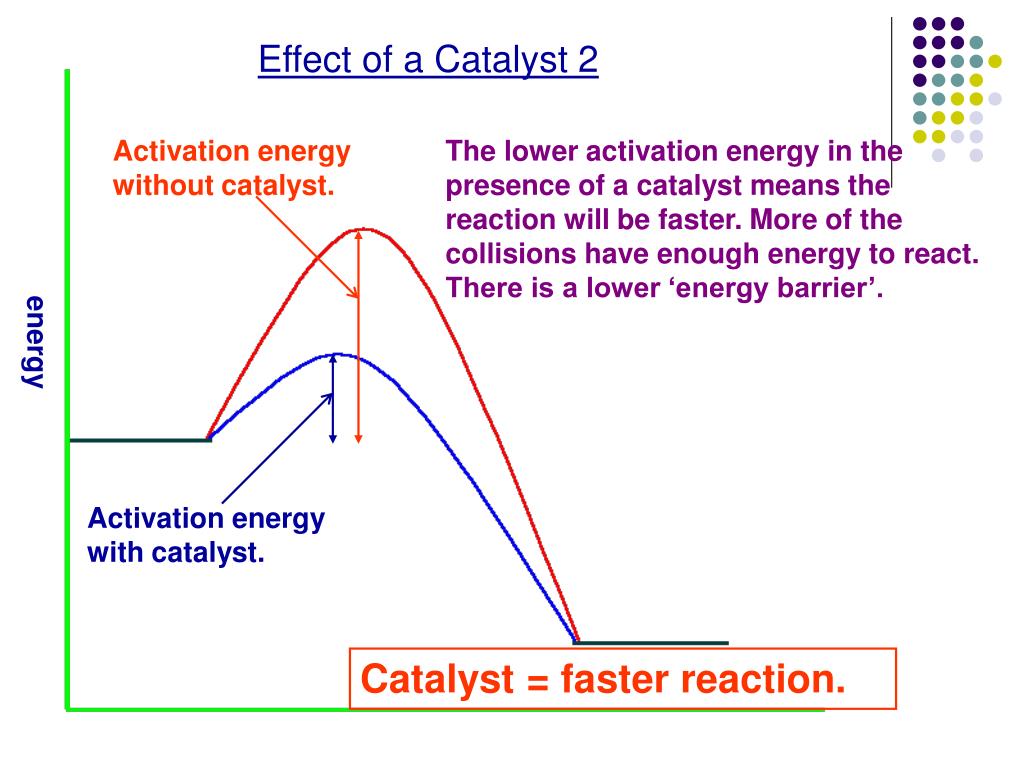
Catalyst Effect On Reaction Rate . Effect of catalysts on rate of reaction. A catalyst does not actually become part of the. Heterogeneous catalysts are in a different phase from the reactants. This page explains how adding a catalyst affects the rate of a reaction. Controlling the ways catalysts work is essential to. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. To investigate the effect of different solids on the catalytic decomposition. Catalysts increase the rate of reactions. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. See examples, diagrams and explanations of collision theory and maxwell.
from www.slideserve.com
See examples, diagrams and explanations of collision theory and maxwell. Effect of catalysts on rate of reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. A catalyst does not actually become part of the. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Heterogeneous catalysts are in a different phase from the reactants. Controlling the ways catalysts work is essential to.
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint
Catalyst Effect On Reaction Rate Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Controlling the ways catalysts work is essential to. See examples, diagrams and explanations of collision theory and maxwell. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. Effect of catalysts on rate of reaction. Catalysts increase the rate of reactions. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. To investigate the effect of different solids on the catalytic decomposition. Heterogeneous catalysts are in a different phase from the reactants. A catalyst does not actually become part of the. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. This page explains how adding a catalyst affects the rate of a reaction.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Catalyst Effect On Reaction Rate Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Heterogeneous catalysts are in a different phase from the reactants. To investigate the effect of different solids on the catalytic decomposition. Controlling the ways catalysts work is essential to. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects. Catalyst Effect On Reaction Rate.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Catalyst Effect On Reaction Rate This page explains how adding a catalyst affects the rate of a reaction. See examples, diagrams and explanations of collision theory and maxwell. A catalyst does not actually become part of the. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. We describe as ‘reversible’ a bidirectional catalyst that. Catalyst Effect On Reaction Rate.
From writeatopic.com
What is the effect of a catalyst on the rate of a reaction? Write A Topic Catalyst Effect On Reaction Rate Controlling the ways catalysts work is essential to. This page explains how adding a catalyst affects the rate of a reaction. Effect of catalysts on rate of reaction. To investigate the effect of different solids on the catalytic decomposition. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Heterogeneous. Catalyst Effect On Reaction Rate.
From exoigvwpw.blob.core.windows.net
The Effect Of Catalysts On Rate Of Reaction at Clyde Berry blog Catalyst Effect On Reaction Rate A catalyst does not actually become part of the. Effect of catalysts on rate of reaction. This page explains how adding a catalyst affects the rate of a reaction. To investigate the effect of different solids on the catalytic decomposition. Heterogeneous catalysts are in a different phase from the reactants. Describe how changing the temperature, concentration of a reactant, or. Catalyst Effect On Reaction Rate.
From www.youtube.com
Effect of Catalyst on Rate of Reaction Chemical Chemistry Catalyst Effect On Reaction Rate A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. This page explains how adding a catalyst affects the rate of a reaction. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. Controlling the ways catalysts work is essential. Catalyst Effect On Reaction Rate.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Catalyst Effect On Reaction Rate To investigate the effect of different solids on the catalytic decomposition. Heterogeneous catalysts are in a different phase from the reactants. See examples, diagrams and explanations of collision theory and maxwell. Controlling the ways catalysts work is essential to. Catalysts increase the rate of reactions. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy.. Catalyst Effect On Reaction Rate.
From www.researchgate.net
Effect of catalyst loading on average reaction rate (TOC initial Catalyst Effect On Reaction Rate Effect of catalysts on rate of reaction. Controlling the ways catalysts work is essential to. To investigate the effect of different solids on the catalytic decomposition. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. See examples, diagrams and explanations of collision theory and maxwell. This page explains how. Catalyst Effect On Reaction Rate.
From telegra.ph
Describe effect catalyst activation energy reaction rate Telegraph Catalyst Effect On Reaction Rate Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. This page explains how adding a catalyst affects the rate of a reaction. Heterogeneous catalysts are in a different phase from the reactants. Catalysts increase the rate of reactions. A catalyst is a substance that increases the rate of a chemical reaction without being used. Catalyst Effect On Reaction Rate.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction Catalyst Effect On Reaction Rate A catalyst does not actually become part of the. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. This page explains how adding a catalyst affects the rate of a reaction. See examples, diagrams and explanations of collision theory and maxwell. Describe how changing the temperature, concentration of a reactant, or surface area of. Catalyst Effect On Reaction Rate.
From chemistry.stackexchange.com
physical chemistry Which diagram shows the effect of catalysis on Catalyst Effect On Reaction Rate Controlling the ways catalysts work is essential to. This page explains how adding a catalyst affects the rate of a reaction. See examples, diagrams and explanations of collision theory and maxwell. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Heterogeneous catalysts are in a different phase from the. Catalyst Effect On Reaction Rate.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Effect On Reaction Rate To investigate the effect of different solids on the catalytic decomposition. Controlling the ways catalysts work is essential to. Heterogeneous catalysts are in a different phase from the reactants. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalysts increase the rate of reactions. A catalyst is a substance that can help the reactants. Catalyst Effect On Reaction Rate.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Catalyst Effect On Reaction Rate This page explains how adding a catalyst affects the rate of a reaction. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. A catalyst is a substance that can help the reactants in a. Catalyst Effect On Reaction Rate.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Effect On Reaction Rate Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. To investigate the effect of different solids on the catalytic decomposition. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with. Catalyst Effect On Reaction Rate.
From loevndwvy.blob.core.windows.net
A Catalyst Increase The Rate Of A Reaction By at Carey Rains blog Catalyst Effect On Reaction Rate Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Controlling the ways catalysts work is essential to. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Catalysts increase the rate of reactions. This page explains how adding a catalyst affects the rate of. Catalyst Effect On Reaction Rate.
From www.youtube.com
29) Effect of Catalyst on Rate of the reaction Activation energy Catalyst Effect On Reaction Rate Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. See examples, diagrams and explanations of collision theory and maxwell. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance that increases the rate of a. Catalyst Effect On Reaction Rate.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Effect On Reaction Rate A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. To investigate the effect of different solids on the catalytic decomposition. This page explains how adding a catalyst affects the rate of a reaction. A catalyst does not actually become part of the. See examples, diagrams and explanations of collision. Catalyst Effect On Reaction Rate.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6050384 Catalyst Effect On Reaction Rate Controlling the ways catalysts work is essential to. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst does not actually become part of the. See examples, diagrams and explanations of collision theory and maxwell. Describe. Catalyst Effect On Reaction Rate.
From study.com
Effect of Catalysts on Rates of Reaction Video & Lesson Transcript Catalyst Effect On Reaction Rate To investigate the effect of different solids on the catalytic decomposition. Controlling the ways catalysts work is essential to. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. This page explains how adding a catalyst affects the rate of a reaction. We describe as ‘reversible’ a bidirectional catalyst that. Catalyst Effect On Reaction Rate.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalyst Effect On Reaction Rate Catalysts increase the rate of reactions. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. This page explains how adding a catalyst affects the rate of a reaction. A catalyst does not actually become part of the. To investigate the effect of different solids on the catalytic decomposition. Describe how changing the temperature, concentration. Catalyst Effect On Reaction Rate.
From studylib.net
Effects of temperature and catalyst on reaction rate Catalyst Effect On Reaction Rate See examples, diagrams and explanations of collision theory and maxwell. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Controlling the ways catalysts work is essential to. A catalyst does not actually become part of the. This page explains how adding a catalyst affects the rate of a reaction. Catalysts increase the rate of. Catalyst Effect On Reaction Rate.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Effect On Reaction Rate A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Catalysts increase the rate of reactions. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy.. Catalyst Effect On Reaction Rate.
From www.researchgate.net
Effect of catalyst concentration on the rate of reaction , MSA; , pTSA Catalyst Effect On Reaction Rate To investigate the effect of different solids on the catalytic decomposition. See examples, diagrams and explanations of collision theory and maxwell. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of. Catalyst Effect On Reaction Rate.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Effect On Reaction Rate We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. See examples, diagrams and explanations of collision theory and maxwell. Learn how catalysts speed up reactions by providing an alternative. Catalyst Effect On Reaction Rate.
From www.slideshare.net
1.4 rate of reaction(1.2d)...biology Catalyst Effect On Reaction Rate To investigate the effect of different solids on the catalytic decomposition. Effect of catalysts on rate of reaction. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Heterogeneous catalysts are in a different phase from the reactants. This page explains how adding a catalyst affects the rate of a reaction. We describe as ‘reversible’. Catalyst Effect On Reaction Rate.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Effect On Reaction Rate To investigate the effect of different solids on the catalytic decomposition. Effect of catalysts on rate of reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. This page explains how adding a catalyst affects the rate of a reaction. Heterogeneous catalysts are in a different phase from the reactants.. Catalyst Effect On Reaction Rate.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Catalyst Effect On Reaction Rate Catalysts increase the rate of reactions. Effect of catalysts on rate of reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Learn how catalysts speed up reactions by providing. Catalyst Effect On Reaction Rate.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Catalyst Effect On Reaction Rate Heterogeneous catalysts are in a different phase from the reactants. A catalyst does not actually become part of the. See examples, diagrams and explanations of collision theory and maxwell. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. Controlling the ways catalysts work is essential to. To investigate the. Catalyst Effect On Reaction Rate.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalyst Effect On Reaction Rate See examples, diagrams and explanations of collision theory and maxwell. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Effect of catalysts on rate of reaction. Heterogeneous catalysts are in a different phase from the reactants. A catalyst is a substance that can help the reactants in a chemical. Catalyst Effect On Reaction Rate.
From chemistryguru.com.sg
Rate Concentration Graph for Enzyme Catalysed Reaction Catalyst Effect On Reaction Rate This page explains how adding a catalyst affects the rate of a reaction. To investigate the effect of different solids on the catalytic decomposition. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Heterogeneous catalysts are in a different phase from the reactants. A catalyst is a substance that. Catalyst Effect On Reaction Rate.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Catalyst Effect On Reaction Rate A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. To investigate the effect of. Catalyst Effect On Reaction Rate.
From www.youtube.com
Effect of Catalyst on rate of reaction Chemical YouTube Catalyst Effect On Reaction Rate See examples, diagrams and explanations of collision theory and maxwell. Heterogeneous catalysts are in a different phase from the reactants. To investigate the effect of different solids on the catalytic decomposition. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. This page explains how adding a catalyst affects the rate of a reaction. A. Catalyst Effect On Reaction Rate.
From www.sasadoctor.com
Rate of reaction chemistry igcse Catalyst Effect On Reaction Rate A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. This page explains how adding a catalyst affects the rate of a reaction. To investigate the effect of different solids on the catalytic decomposition. Controlling the ways catalysts work is essential to. Describe how changing the temperature, concentration of a reactant,. Catalyst Effect On Reaction Rate.
From www.youtube.com
Effect of catalyst on rate of reaction YouTube Catalyst Effect On Reaction Rate A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Controlling the ways catalysts work is essential to. Heterogeneous catalysts are in a different phase from the reactants. See examples, diagrams. Catalyst Effect On Reaction Rate.
From www.researchgate.net
Effect of catalyst preparation on reaction rate of simultaneous Catalyst Effect On Reaction Rate Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. To investigate the effect of different solids on the catalytic decomposition. Catalysts increase the rate of reactions. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to. Heterogeneous catalysts are. Catalyst Effect On Reaction Rate.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Effect On Reaction Rate Catalysts increase the rate of reactions. A catalyst does not actually become part of the. Describe how changing the temperature, concentration of a reactant, or surface area of a reaction affects the rate of a. See examples, diagrams and explanations of collision theory and maxwell. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy.. Catalyst Effect On Reaction Rate.