Does Vinegar Soluble In Water . Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. When an element or substance dissolves in water, it is considered soluble. Many people wonder whether vinegar can dissolve in water. The table below provides information on the variation of solubility of different substances (mostly inorganic. The electrostatic attraction between an ion and a. How many of the given substances are soluble in water? The short answer is yes, vinegar does dissolve in water. Use the solubility rules to predict if a compound is soluble,. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. Sugar, petrol, salt, vinegar, sand, oil Vinegar does not dissolve in water. Define and give examples of electrolytes. Vinegar is a dilute solution of. Vinegar is soluble in water.
from pestsource.com
The electrostatic attraction between an ion and a. Sugar, petrol, salt, vinegar, sand, oil Vinegar is soluble in water. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. Vinegar does not dissolve in water. Vinegar is a dilute solution of. The short answer is yes, vinegar does dissolve in water. The table below provides information on the variation of solubility of different substances (mostly inorganic. Many people wonder whether vinegar can dissolve in water. Use the solubility rules to predict if a compound is soluble,.
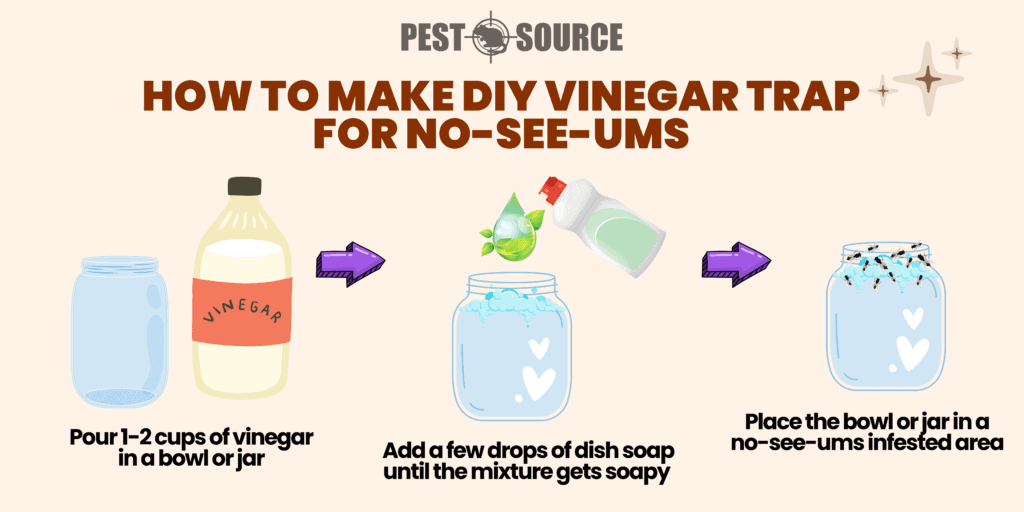
Does Vinegar Kill NoSeeUms? Pest Source
Does Vinegar Soluble In Water Use the solubility rules to predict if a compound is soluble,. Vinegar is soluble in water. Use the solubility rules to predict if a compound is soluble,. The short answer is yes, vinegar does dissolve in water. Vinegar does not dissolve in water. Define and give examples of electrolytes. Vinegar is a dilute solution of. When an element or substance dissolves in water, it is considered soluble. How many of the given substances are soluble in water? Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. The electrostatic attraction between an ion and a. The table below provides information on the variation of solubility of different substances (mostly inorganic. Many people wonder whether vinegar can dissolve in water. Sugar, petrol, salt, vinegar, sand, oil When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar.
From solutionpharmacy.in
Factors Influencing Solubility Solution Parmacy Does Vinegar Soluble In Water Vinegar is soluble in water. Vinegar does not dissolve in water. Use the solubility rules to predict if a compound is soluble,. The short answer is yes, vinegar does dissolve in water. How many of the given substances are soluble in water? When an element or substance dissolves in water, it is considered soluble. Many people wonder whether vinegar can. Does Vinegar Soluble In Water.
From cleanerprofy.com
How Long Does Vinegar Smell Last? CleanerProfy Does Vinegar Soluble In Water The table below provides information on the variation of solubility of different substances (mostly inorganic. How many of the given substances are soluble in water? The short answer is yes, vinegar does dissolve in water. When an element or substance dissolves in water, it is considered soluble. Many people wonder whether vinegar can dissolve in water. Vinegar is a dilute. Does Vinegar Soluble In Water.
From www.youtube.com
Does Apple Cider Vinegar Kill Mold? Damp Solving Water & Mold YouTube Does Vinegar Soluble In Water Use the solubility rules to predict if a compound is soluble,. Vinegar is a dilute solution of. Vinegar does not dissolve in water. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. How many of the given substances are soluble in water? When added to water, vinegar dissolves completely to form a homogeneous mixture. Does Vinegar Soluble In Water.
From www.pinterest.com
This is a cool science experiment. Place a piece of chalk in water Does Vinegar Soluble In Water The electrostatic attraction between an ion and a. Vinegar does not dissolve in water. Many people wonder whether vinegar can dissolve in water. The table below provides information on the variation of solubility of different substances (mostly inorganic. When an element or substance dissolves in water, it is considered soluble. When added to water, vinegar dissolves completely to form a. Does Vinegar Soluble In Water.
From www.youtube.com
Can Drinking Vinegar Water Really Make You Alkaline? YouTube Does Vinegar Soluble In Water Use the solubility rules to predict if a compound is soluble,. Define and give examples of electrolytes. How many of the given substances are soluble in water? The short answer is yes, vinegar does dissolve in water. Sugar, petrol, salt, vinegar, sand, oil Vinegar is soluble in water. Many people wonder whether vinegar can dissolve in water. Water and other. Does Vinegar Soluble In Water.
From www.youtube.com
Experiment Does Chalk dissolve in Water,Vinegar And Lemon or not Does Vinegar Soluble In Water Vinegar is soluble in water. Use the solubility rules to predict if a compound is soluble,. The electrostatic attraction between an ion and a. Sugar, petrol, salt, vinegar, sand, oil Vinegar is a dilute solution of. Vinegar does not dissolve in water. When an element or substance dissolves in water, it is considered soluble. When added to water, vinegar dissolves. Does Vinegar Soluble In Water.
From msk-ingredients.com
Balsamic Vinegar (Natural) Flavour Burst (water soluble), 100ml Does Vinegar Soluble In Water When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. The electrostatic attraction between an ion and a. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. Sugar, petrol, salt, vinegar, sand, oil Vinegar is a dilute solution of. Define and give examples of electrolytes. The short answer. Does Vinegar Soluble In Water.
From www.mytidycorner.com
Vinegar to Water Ratio for Cleaning Different Surfaces and Things Does Vinegar Soluble In Water Vinegar does not dissolve in water. The table below provides information on the variation of solubility of different substances (mostly inorganic. Define and give examples of electrolytes. Vinegar is soluble in water. Vinegar is a dilute solution of. Many people wonder whether vinegar can dissolve in water. How many of the given substances are soluble in water? The electrostatic attraction. Does Vinegar Soluble In Water.
From askfilo.com
7. Are vinegar and lemon juice soluble or insoluble in water? Filo Does Vinegar Soluble In Water Many people wonder whether vinegar can dissolve in water. When an element or substance dissolves in water, it is considered soluble. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. Define and give examples of electrolytes. The electrostatic attraction between an ion and a. Use the solubility rules to predict if a compound. Does Vinegar Soluble In Water.
From yummykitchentv.com
What is Vinegar ? What does it actually do to food? Yummy Kitchen Does Vinegar Soluble In Water Define and give examples of electrolytes. Many people wonder whether vinegar can dissolve in water. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. The table below provides information on the variation of solubility of different substances (mostly inorganic. Use the solubility rules to predict if a compound is soluble,. Vinegar is soluble in. Does Vinegar Soluble In Water.
From www.numerade.com
SOLVED ALCOHOLS, PHENOLS CARBOXYLIC ACIDS Solubility in Water (cross Does Vinegar Soluble In Water Define and give examples of electrolytes. How many of the given substances are soluble in water? When an element or substance dissolves in water, it is considered soluble. Vinegar is soluble in water. The short answer is yes, vinegar does dissolve in water. The table below provides information on the variation of solubility of different substances (mostly inorganic. Vinegar is. Does Vinegar Soluble In Water.
From familygoody.com
What Ratio Of Vinegar To Water Is Best For Mopping? Does Vinegar Soluble In Water Vinegar is soluble in water. The electrostatic attraction between an ion and a. Vinegar is a dilute solution of. Vinegar does not dissolve in water. Define and give examples of electrolytes. How many of the given substances are soluble in water? The short answer is yes, vinegar does dissolve in water. When added to water, vinegar dissolves completely to form. Does Vinegar Soluble In Water.
From foodstruct.com
Vinegar vs. Water — InDepth Nutrition Comparison Does Vinegar Soluble In Water When an element or substance dissolves in water, it is considered soluble. How many of the given substances are soluble in water? When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. Many people wonder whether vinegar can dissolve in water. Vinegar is soluble in water. Use the solubility rules to predict if a. Does Vinegar Soluble In Water.
From www.youtube.com
Why Salt (NaCl) Does Not Dissolve in Oil Salt and Oil Experiment Is Does Vinegar Soluble In Water Many people wonder whether vinegar can dissolve in water. Sugar, petrol, salt, vinegar, sand, oil Vinegar is a dilute solution of. The short answer is yes, vinegar does dissolve in water. When an element or substance dissolves in water, it is considered soluble. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. Define. Does Vinegar Soluble In Water.
From www.tipsbulletin.com
What You Need to Know about Vinegar & Whether It Can Spoil Does Vinegar Soluble In Water Vinegar does not dissolve in water. When an element or substance dissolves in water, it is considered soluble. Many people wonder whether vinegar can dissolve in water. Sugar, petrol, salt, vinegar, sand, oil How many of the given substances are soluble in water? The electrostatic attraction between an ion and a. When added to water, vinegar dissolves completely to form. Does Vinegar Soluble In Water.
From www.fabhow.com
How to Use Apple Cider Vinegar for Acid Reflux Fab How Does Vinegar Soluble In Water Vinegar does not dissolve in water. The table below provides information on the variation of solubility of different substances (mostly inorganic. Use the solubility rules to predict if a compound is soluble,. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. When an element or substance dissolves in water, it is considered soluble.. Does Vinegar Soluble In Water.
From www.mytidycorner.com
Vinegar to Water Ratio for Cleaning Different Surfaces and Things Does Vinegar Soluble In Water The table below provides information on the variation of solubility of different substances (mostly inorganic. Sugar, petrol, salt, vinegar, sand, oil The electrostatic attraction between an ion and a. Use the solubility rules to predict if a compound is soluble,. Define and give examples of electrolytes. When an element or substance dissolves in water, it is considered soluble. Vinegar does. Does Vinegar Soluble In Water.
From www.youtube.com
Baking Soda & Vinegar Reaction vs. Temperature YouTube Does Vinegar Soluble In Water Use the solubility rules to predict if a compound is soluble,. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. How many of the given substances are soluble in water? When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. The table below provides information on the variation. Does Vinegar Soluble In Water.
From studylibraryintroit.z14.web.core.windows.net
Experiment Using Baking Soda Does Vinegar Soluble In Water The short answer is yes, vinegar does dissolve in water. The electrostatic attraction between an ion and a. Use the solubility rules to predict if a compound is soluble,. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. Vinegar is soluble in water. The table below provides information on the variation of solubility of. Does Vinegar Soluble In Water.
From learninglibandres123.z19.web.core.windows.net
Baking Soda And Vinegar Experiment Worksheet Does Vinegar Soluble In Water The electrostatic attraction between an ion and a. The table below provides information on the variation of solubility of different substances (mostly inorganic. Vinegar is soluble in water. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. Sugar, petrol, salt, vinegar, sand, oil Many people wonder whether vinegar can dissolve in water. The. Does Vinegar Soluble In Water.
From www.pinterest.com.au
What Is the VinegartoWater Ratio for Cleaning? Hunker Vinegar and Does Vinegar Soluble In Water Use the solubility rules to predict if a compound is soluble,. Vinegar is soluble in water. When an element or substance dissolves in water, it is considered soluble. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. The table below provides information on the variation of solubility of different substances (mostly inorganic. Vinegar is. Does Vinegar Soluble In Water.
From muisjart.blogspot.com
Is Vinegar Soluble In Water Does Vinegar Soluble In Water The short answer is yes, vinegar does dissolve in water. Vinegar does not dissolve in water. Sugar, petrol, salt, vinegar, sand, oil The table below provides information on the variation of solubility of different substances (mostly inorganic. Vinegar is a dilute solution of. When an element or substance dissolves in water, it is considered soluble. How many of the given. Does Vinegar Soluble In Water.
From www.theinstantpottable.com
Does Apple Cider Vinegar Go Bad? The Instant Pot Table Does Vinegar Soluble In Water Vinegar is soluble in water. Vinegar is a dilute solution of. How many of the given substances are soluble in water? Vinegar does not dissolve in water. When an element or substance dissolves in water, it is considered soluble. The short answer is yes, vinegar does dissolve in water. Sugar, petrol, salt, vinegar, sand, oil Define and give examples of. Does Vinegar Soluble In Water.
From inthewash.co.uk
Does Vinegar Destroy Rubber? Does Vinegar Soluble In Water The electrostatic attraction between an ion and a. Sugar, petrol, salt, vinegar, sand, oil When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. The short answer is yes, vinegar does dissolve in water. Vinegar does not dissolve in water. Vinegar is soluble in water. Define and give examples of electrolytes. The table below. Does Vinegar Soluble In Water.
From slideplayer.com
Edward Wen Solutions. 2 Learning Solutions (applying Does Vinegar Soluble In Water Vinegar does not dissolve in water. The electrostatic attraction between an ion and a. When an element or substance dissolves in water, it is considered soluble. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. Sugar, petrol, salt, vinegar, sand, oil Define and give examples of electrolytes. The table below provides information on the. Does Vinegar Soluble In Water.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Does Vinegar Soluble In Water Use the solubility rules to predict if a compound is soluble,. The short answer is yes, vinegar does dissolve in water. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. The electrostatic attraction between an ion and a. Sugar, petrol, salt, vinegar, sand, oil When added to water, vinegar dissolves completely to form a. Does Vinegar Soluble In Water.
From woodworkly.com
Can You Use Vinegar On Wood? (5 EASY Steps!) WoodWorkly Does Vinegar Soluble In Water Sugar, petrol, salt, vinegar, sand, oil How many of the given substances are soluble in water? Use the solubility rules to predict if a compound is soluble,. Vinegar does not dissolve in water. Define and give examples of electrolytes. The table below provides information on the variation of solubility of different substances (mostly inorganic. Water and other polar molecules are. Does Vinegar Soluble In Water.
From slideplayer.com
AQUEOUS SYSTEMS. ppt download Does Vinegar Soluble In Water Use the solubility rules to predict if a compound is soluble,. The short answer is yes, vinegar does dissolve in water. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. The table below provides information on the variation of solubility of different substances (mostly inorganic. When an element or substance dissolves in water,. Does Vinegar Soluble In Water.
From sciencenotes.org
Egg in Vinegar Experiment Make a Rubber Egg Does Vinegar Soluble In Water The table below provides information on the variation of solubility of different substances (mostly inorganic. Use the solubility rules to predict if a compound is soluble,. Vinegar does not dissolve in water. The electrostatic attraction between an ion and a. The short answer is yes, vinegar does dissolve in water. Many people wonder whether vinegar can dissolve in water. When. Does Vinegar Soluble In Water.
From www.fabricfits.com
Does Vinegar Stain Clothes? How To Remove? Does Vinegar Soluble In Water The table below provides information on the variation of solubility of different substances (mostly inorganic. How many of the given substances are soluble in water? The short answer is yes, vinegar does dissolve in water. Use the solubility rules to predict if a compound is soluble,. The electrostatic attraction between an ion and a. When an element or substance dissolves. Does Vinegar Soluble In Water.
From inthewash.co.uk
Does Vinegar Help When Doing Laundry with Hard Water? Does Vinegar Soluble In Water When an element or substance dissolves in water, it is considered soluble. The electrostatic attraction between an ion and a. Sugar, petrol, salt, vinegar, sand, oil Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. Define and give examples of electrolytes. When added to water, vinegar dissolves completely to form a homogeneous mixture due. Does Vinegar Soluble In Water.
From www.youtube.com
Baking soda and vinegar reaction. ⚗️ 3 Experiments to do with kids ⚗️ Does Vinegar Soluble In Water Many people wonder whether vinegar can dissolve in water. Sugar, petrol, salt, vinegar, sand, oil Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. The electrostatic attraction between an ion and a. Use the solubility rules to predict. Does Vinegar Soluble In Water.
From pestsource.com
Does Vinegar Kill NoSeeUms? Pest Source Does Vinegar Soluble In Water The table below provides information on the variation of solubility of different substances (mostly inorganic. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. Water and other polar molecules are attracted to ions, as shown in figure 7.5.2 7.5. How many of the given substances are soluble in water? The electrostatic attraction between. Does Vinegar Soluble In Water.
From www.epicurious.com
Does Vinegar Go Bad? Epicurious Does Vinegar Soluble In Water The table below provides information on the variation of solubility of different substances (mostly inorganic. How many of the given substances are soluble in water? The electrostatic attraction between an ion and a. Vinegar is a dilute solution of. Many people wonder whether vinegar can dissolve in water. Use the solubility rules to predict if a compound is soluble,. The. Does Vinegar Soluble In Water.
From www.youtube.com
Can Liquids Dissolve in Water? YouTube Does Vinegar Soluble In Water How many of the given substances are soluble in water? The table below provides information on the variation of solubility of different substances (mostly inorganic. Use the solubility rules to predict if a compound is soluble,. When added to water, vinegar dissolves completely to form a homogeneous mixture due to its polar. Many people wonder whether vinegar can dissolve in. Does Vinegar Soluble In Water.