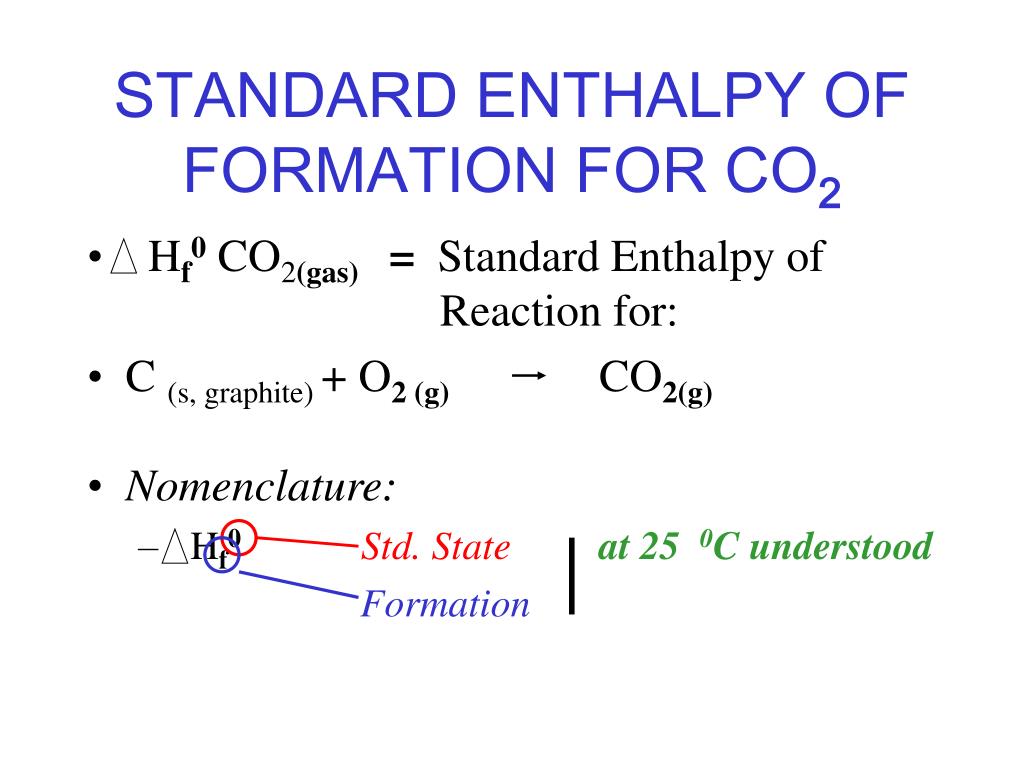
Standard Enthalpy Of Formation Cr . The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard heats and free energies of formation and absolute entropies of elements and. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
from www.slideserve.com
193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard heats and free energies of formation and absolute entropies of elements and. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of.
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation
Standard Enthalpy Of Formation Cr The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standard heats and free energies of formation and absolute entropies of elements and. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
From www.toppr.com
Calculate the standard enthalpy of the reactions 2C (graphite) + 3 H2(g Standard Enthalpy Of Formation Cr The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard heats and free energies of formation and absolute entropies of elements and. The. Standard Enthalpy Of Formation Cr.
From www.toppr.com
The standard molar enthalpies of formation of cyclohexane (I) and Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy. Standard Enthalpy Of Formation Cr.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Cr 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one. Standard Enthalpy Of Formation Cr.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy. Standard Enthalpy Of Formation Cr.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Enthalpy Of Formation Cr.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Cr The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Enthalpy Of Formation Cr.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Cr The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standard heats and free energies of formation and absolute entropies of elements and. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The. Standard Enthalpy Of Formation Cr.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation Cr.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.. Standard Enthalpy Of Formation Cr.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is. Standard Enthalpy Of Formation Cr.
From schoolworkhelper.net
Standard Enthalpies of Formation, ΔH°f SchoolWorkHelper Standard Enthalpy Of Formation Cr 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard heats and free energies of formation and absolute entropies of elements and. The standard enthalpy. Standard Enthalpy Of Formation Cr.
From www.researchgate.net
Reaction enthalpies, and standard enthalpies of formation of alkaline Standard Enthalpy Of Formation Cr The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the standard enthalpy of formation is measured in units of energy per. Standard Enthalpy Of Formation Cr.
From www.numerade.com
SOLVEDUse standard enthalpies of formation to calculate ΔHrxn^∘ for Standard Enthalpy Of Formation Cr The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of. Standard Enthalpy Of Formation Cr.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation is defined as. Standard Enthalpy Of Formation Cr.
From www.chegg.com
Solved Table 6.2. Selected Standard Molar Enthalpies of Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation Cr.
From www.researchgate.net
(PDF) Standard enthalpies of formation of Li, Na, K, and Cs thiolates Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation Cr.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole. Standard Enthalpy Of Formation Cr.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Cr The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these tables include heat of. Standard Enthalpy Of Formation Cr.
From www.chegg.com
Solved Which processes in the table have enthalpies of Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Hgo cr,red −90.79. Standard Enthalpy Of Formation Cr.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Cr The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is. Standard Enthalpy Of Formation Cr.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Cr 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies. Standard Enthalpy Of Formation Cr.
From www.chegg.com
Solved Calculate the Standard Enthalpy of Formation of NOCl Standard Enthalpy Of Formation Cr Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0.. Standard Enthalpy Of Formation Cr.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of. Standard Enthalpy Of Formation Cr.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Cr Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to. Standard Enthalpy Of Formation Cr.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Cr The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule. Standard Enthalpy Of Formation Cr.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard heats and free energies of formation and absolute entropies of elements and. The standard enthalpy of formation is defined as the change. Standard Enthalpy Of Formation Cr.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation Cr.
From www.youtube.com
standard_enthalpies_of_formation YouTube Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation (δh0f). Standard Enthalpy Of Formation Cr.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Cr The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of. Standard Enthalpy Of Formation Cr.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of. Standard Enthalpy Of Formation Cr.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole.. Standard Enthalpy Of Formation Cr.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Cr The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of. Standard Enthalpy Of Formation Cr.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the. Standard Enthalpy Of Formation Cr.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Cr Hgo cr,red −90.79 70.3 ∗the standard entropy of the h+(aq) ion is defined to be 0. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard heats and free energies of formation and absolute entropies of elements and. 193 rows the standard enthalpy of. Standard Enthalpy Of Formation Cr.
From www.chegg.com
Using enthalpies of formation (Appendix C below, can Standard Enthalpy Of Formation Cr Standard heats and free energies of formation and absolute entropies of elements and. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation Cr.