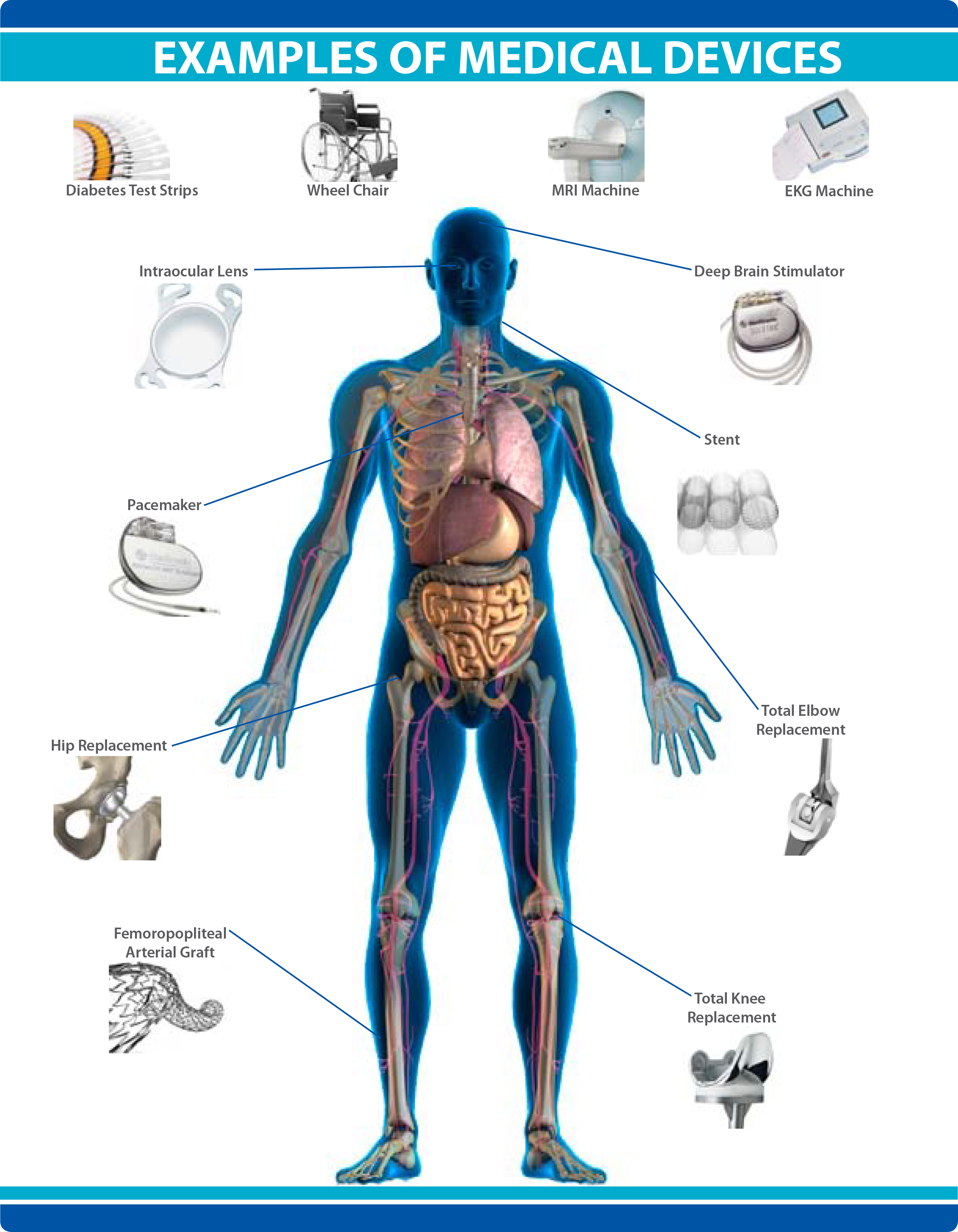
What Is A Class 3 Medical Device . The following tool will assist. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. Class iii general controls and premarket approval. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. The class to which your device is assigned determines, among other things, the type of. Medical devices are classified according to the level of harm they may pose to users or patients. The medical devices regulation requires medical devices to be classified into one of the four classes: Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices.
from lifechanginginnovation.org
The class to which your device is assigned determines, among other things, the type of. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. The following tool will assist. Class iii general controls and premarket approval. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices are classified according to the level of harm they may pose to users or patients. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The medical devices regulation requires medical devices to be classified into one of the four classes:
What is a Medical Device? Life Changing Innovation
What Is A Class 3 Medical Device The following tool will assist. Medical devices are classified according to the level of harm they may pose to users or patients. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Class iii general controls and premarket approval. The class to which your device is assigned determines, among other things, the type of. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The medical devices regulation requires medical devices to be classified into one of the four classes: The following tool will assist.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations What Is A Class 3 Medical Device The medical devices regulation requires medical devices to be classified into one of the four classes: The following tool will assist. Medical devices are classified according to the level of harm they may pose to users or patients. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii. What Is A Class 3 Medical Device.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group What Is A Class 3 Medical Device Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The following tool will assist. Medical devices are classified according to the level of harm they may pose to users or patients. The class to which your. What Is A Class 3 Medical Device.
From es.slideshare.net
Regulation of Medical Devices in US What Is A Class 3 Medical Device As the class increases, so does the risk to the individual, and so does the required testing to ensure that. The medical devices regulation requires medical devices to be classified into one of the four classes: The following tool will assist. Class iii general controls and premarket approval. The class to which your device is assigned determines, among other things,. What Is A Class 3 Medical Device.
From ramtechno.com
What Are the Three FDA Classes for Medical Devices? RAM Technologies What Is A Class 3 Medical Device The class to which your device is assigned determines, among other things, the type of. Class iii general controls and premarket approval. The following tool will assist. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. The medical devices regulation requires medical devices to be classified into one of the four. What Is A Class 3 Medical Device.
From omcmedical.com
Classification of Medical Devices Based on UK MDR 2002 What Is A Class 3 Medical Device The medical devices regulation requires medical devices to be classified into one of the four classes: The following tool will assist. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Class iii general controls and premarket approval. Medical devices with a higher risk profile or lower perceived benefits are. What Is A Class 3 Medical Device.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is A Class 3 Medical Device As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices are classified according to the level of harm they may pose to users or patients. The following tool will assist. Any medical device approved by the fda center for devices and radiological health is classified as either class. What Is A Class 3 Medical Device.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is A Class 3 Medical Device The medical devices regulation requires medical devices to be classified into one of the four classes: Medical devices are classified according to the level of harm they may pose to users or patients. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s. What Is A Class 3 Medical Device.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co What Is A Class 3 Medical Device Class iii general controls and premarket approval. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices are classified according to the level of harm they may pose to users or patients. The class to which your device is assigned determines, among other things, the type of. The. What Is A Class 3 Medical Device.
From www.regdesk.co
What is a class III medical device? RegDesk What Is A Class 3 Medical Device Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. The medical devices regulation requires medical devices to be. What Is A Class 3 Medical Device.
From medicaldevicehq.com
Different classifications rules for medical device software An What Is A Class 3 Medical Device The class to which your device is assigned determines, among other things, the type of. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. The medical devices regulation requires medical devices. What Is A Class 3 Medical Device.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero What Is A Class 3 Medical Device Medical devices are classified according to the level of harm they may pose to users or patients. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the. What Is A Class 3 Medical Device.
From www.volersystems.com
Are You Making a Medical Device? Voler Systems What Is A Class 3 Medical Device Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The following tool will assist. The medical devices regulation requires medical devices to be classified into one of the four classes: As the class increases, so does. What Is A Class 3 Medical Device.
From www.afcinternationalllc.com
Importing Medical Devices Three Classes You Should Know What Is A Class 3 Medical Device Class iii general controls and premarket approval. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. Medical devices are classified according to the level of harm they may pose to users or patients. The following tool will assist. Any medical device approved by the fda center for devices and radiological health. What Is A Class 3 Medical Device.
From www.presentationeze.com
FDA medical device classification PresentationEZE What Is A Class 3 Medical Device Class iii general controls and premarket approval. The medical devices regulation requires medical devices to be classified into one of the four classes: As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices are classified according to the level of harm they may pose to users or patients.. What Is A Class 3 Medical Device.
From www.vrogue.co
What Is The Fda Definition Of A Medical Device Class vrogue.co What Is A Class 3 Medical Device The following tool will assist. The class to which your device is assigned determines, among other things, the type of. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. The medical devices regulation requires medical devices to be classified into one of the four classes: Medical devices with a. What Is A Class 3 Medical Device.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co What Is A Class 3 Medical Device Medical devices are classified according to the level of harm they may pose to users or patients. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The medical devices regulation requires medical devices to be classified. What Is A Class 3 Medical Device.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is A Class 3 Medical Device Medical devices are classified according to the level of harm they may pose to users or patients. The following tool will assist. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices.. What Is A Class 3 Medical Device.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX What Is A Class 3 Medical Device Medical devices are classified according to the level of harm they may pose to users or patients. Class iii general controls and premarket approval. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. The following tool will assist. As the class increases, so does the risk to the individual, and so. What Is A Class 3 Medical Device.
From www.pacificbridgemedical.com
Medical Device Submission Process in Singapore Infographic What Is A Class 3 Medical Device As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices are classified according to the level of harm they may pose to users or patients. The following tool will assist. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices.. What Is A Class 3 Medical Device.
From laegemiddelstyrelsen.dk
Medical devices What Is A Class 3 Medical Device The medical devices regulation requires medical devices to be classified into one of the four classes: The class to which your device is assigned determines, among other things, the type of. Class iii general controls and premarket approval. The following tool will assist. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical. What Is A Class 3 Medical Device.
From angelanjohnson.com
Medical Devices Angela N Johnson What Is A Class 3 Medical Device Medical devices are classified according to the level of harm they may pose to users or patients. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The class to which your device is assigned determines, among. What Is A Class 3 Medical Device.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained What Is A Class 3 Medical Device As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. Medical devices with a higher risk profile. What Is A Class 3 Medical Device.
From www.rimsys.io
Class III medical devices in the United States What Is A Class 3 Medical Device As the class increases, so does the risk to the individual, and so does the required testing to ensure that. The class to which your device is assigned determines, among other things, the type of. The medical devices regulation requires medical devices to be classified into one of the four classes: Any medical device approved by the fda center for. What Is A Class 3 Medical Device.
From lifechanginginnovation.org
What is a Medical Device? Life Changing Innovation What Is A Class 3 Medical Device The medical devices regulation requires medical devices to be classified into one of the four classes: As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on. What Is A Class 3 Medical Device.
From clin-r.com
Labels for Medical Devices Clin R What Is A Class 3 Medical Device Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The class to which your device is assigned determines, among other things, the type of. Class iii general controls and premarket approval. The medical devices regulation requires. What Is A Class 3 Medical Device.
From cortex-design.com
Cortex Design • What's My FDA Medical Device Classification? What Is A Class 3 Medical Device Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. As the class increases, so does the risk to. What Is A Class 3 Medical Device.
From www.grantsformedical.com
What are Class 3 Medical Devices? Grants for Medical What Is A Class 3 Medical Device As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices are classified according to the level of harm they may pose to users or patients. The medical devices regulation requires medical devices to be classified into one of the four classes: Any medical device approved by the fda. What Is A Class 3 Medical Device.
From operonstrategist.com
Classifying a Class III Medical Device Process) Operon What Is A Class 3 Medical Device The medical devices regulation requires medical devices to be classified into one of the four classes: Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The class to which your device is assigned determines, among other. What Is A Class 3 Medical Device.
From www.grantsformedical.com
What are Class 3 Medical Devices? Grants for Medical What Is A Class 3 Medical Device The class to which your device is assigned determines, among other things, the type of. Medical devices are classified according to the level of harm they may pose to users or patients. As the class increases, so does the risk to the individual, and so does the required testing to ensure that. The following tool will assist. The medical devices. What Is A Class 3 Medical Device.
From dxocmqwwv.blob.core.windows.net
What Is Considered A Medical Device By The Fda at Lucas Cunningham blog What Is A Class 3 Medical Device As the class increases, so does the risk to the individual, and so does the required testing to ensure that. Medical devices with a higher risk profile or lower perceived benefits are classified as class iii medical devices. Class iii general controls and premarket approval. The class to which your device is assigned determines, among other things, the type of.. What Is A Class 3 Medical Device.
From emmainternational.com
Classifying Medical Devices under EU MDR What Is A Class 3 Medical Device Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. Class iii general controls and premarket approval. As the class increases, so does the risk to the individual, and so does the required testing to ensure that.. What Is A Class 3 Medical Device.
From coastbiomed.com
Blog Coast Biomedical Equipment What Is A Class 3 Medical Device The following tool will assist. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. Class iii general controls and premarket approval. Medical devices with a higher risk profile or lower perceived benefits are classified as class. What Is A Class 3 Medical Device.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market What Is A Class 3 Medical Device Medical devices are classified according to the level of harm they may pose to users or patients. Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. The following tool will assist. Class iii general controls and. What Is A Class 3 Medical Device.
From patientguard.com
Medical Devices Classification UK Class I, II and III What Is A Class 3 Medical Device Any medical device approved by the fda center for devices and radiological health is classified as either class i, ii, or iii depending on the new device’s risk, invasiveness, and impact on the. Class iii general controls and premarket approval. The class to which your device is assigned determines, among other things, the type of. The medical devices regulation requires. What Is A Class 3 Medical Device.
From blog.chino.io
What MDR class is my eHealth app? What Is A Class 3 Medical Device The medical devices regulation requires medical devices to be classified into one of the four classes: Class iii general controls and premarket approval. Medical devices are classified according to the level of harm they may pose to users or patients. As the class increases, so does the risk to the individual, and so does the required testing to ensure that.. What Is A Class 3 Medical Device.