Heating Curve For Sodium . a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). heating curve for sodium chloride. Temperature) were correlated with heating curves (plots of temperature vs. These two types of plots provide. How to calculate enthalpy changes in heating & cooling | crash chemistry Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. phase diagrams (plots of pressure vs. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion.
from www.doubtnut.com
Temperature) were correlated with heating curves (plots of temperature vs. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two types of plots provide. phase diagrams (plots of pressure vs. How to calculate enthalpy changes in heating & cooling | crash chemistry heating curve for sodium chloride. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does.
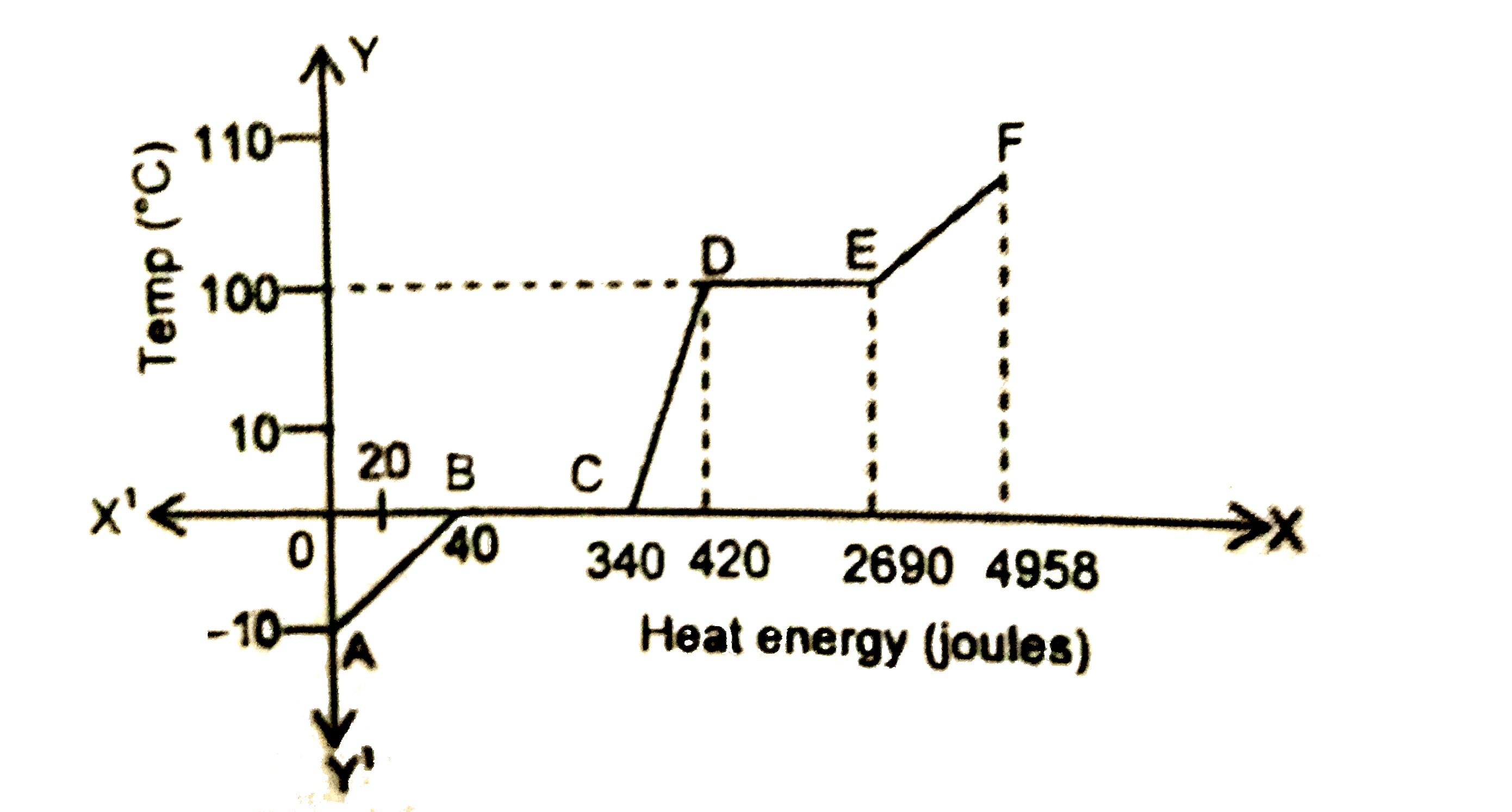
The heating curve of a particular substance in solid state is a shown
Heating Curve For Sodium a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Temperature) were correlated with heating curves (plots of temperature vs. How to calculate enthalpy changes in heating & cooling | crash chemistry the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. heating curve for sodium chloride. These two types of plots provide. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). phase diagrams (plots of pressure vs.
From spmphysics.onlinetuition.com.my
The Heating Curve SPM Physics Form 4/Form 5 Revision Notes Heating Curve For Sodium phase diagrams (plots of pressure vs. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. a plot of the temperature. Heating Curve For Sodium.
From www.researchgate.net
DSC cooling curves and heating curves of liquid aqueous solutions Heating Curve For Sodium phase diagrams (plots of pressure vs. heating curve for sodium chloride. How to calculate enthalpy changes in heating & cooling | crash chemistry These two types of plots provide. Temperature) were correlated with heating curves (plots of temperature vs. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub). Heating Curve For Sodium.
From www.numerade.com
SOLVED Heating/Cooling Curve for Sodium Chloride 6. Label the letters Heating Curve For Sodium These two types of plots provide. phase diagrams (plots of pressure vs. heating curve for sodium chloride. How to calculate enthalpy changes in heating & cooling | crash chemistry Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. Temperature) were correlated with heating curves (plots of temperature vs.. Heating Curve For Sodium.
From brainly.in
Please help draw the heating curve of sodium with the units of Heating Curve For Sodium Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. Temperature) were correlated with heating curves (plots of temperature vs. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two types of plots provide. the amount of. Heating Curve For Sodium.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curve For Sodium heating curve for sodium chloride. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. These two types of plots. Heating Curve For Sodium.
From www.gauthmath.com
Solved True or False The solid form of NaCl has more energy Heating Curve For Sodium the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. These two types of plots provide. Temperature) were correlated with heating curves (plots. Heating Curve For Sodium.
From pdfslide.net
(PDF) Heating and Cooling Curve of Sodium Thiosulfaterkeenan.cmswiki Heating Curve For Sodium the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. How to calculate enthalpy changes in heating & cooling | crash chemistry heating curve for sodium chloride. These two types of plots provide. a plot of the temperature versus the. Heating Curve For Sodium.
From quizizz.com
Heating Curve Graphs Chemistry Quizizz Heating Curve For Sodium Temperature) were correlated with heating curves (plots of temperature vs. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). heating curve for sodium chloride. How to calculate enthalpy changes in heating & cooling | crash chemistry These two types of plots provide. phase diagrams (plots of. Heating Curve For Sodium.
From brainly.in
Q2. The given graph shows the heating curve for a pure substance,the Heating Curve For Sodium a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. phase diagrams (plots of pressure vs. How to calculate enthalpy. Heating Curve For Sodium.
From www.slideshare.net
Properties of Matter Heating Curve For Sodium the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. a plot of the temperature versus the amount of heat added is. Heating Curve For Sodium.
From www.doubtnut.com
The heating curve of a particular substance in solid state is a shown Heating Curve For Sodium phase diagrams (plots of pressure vs. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Temperature) were correlated with heating curves (plots of temperature vs. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100. Heating Curve For Sodium.
From www.albert.io
Heating Curve and Specific Heat Chemistry Practice Albert Heating Curve For Sodium Temperature) were correlated with heating curves (plots of temperature vs. How to calculate enthalpy changes in heating & cooling | crash chemistry Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub). Heating Curve For Sodium.
From protonsforbreakfast.wordpress.com
Sodium Acetate first cooling curve Protons for Breakfast Heating Curve For Sodium Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. heating curve for sodium chloride. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two types of plots provide. the amount of energy needed to sublime. Heating Curve For Sodium.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve For Sodium a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two types of plots provide. How to calculate enthalpy changes in heating & cooling | crash chemistry phase diagrams (plots of pressure vs. heating curve for sodium chloride. the amount of energy needed to sublime. Heating Curve For Sodium.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve For Sodium heating curve for sodium chloride. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. How to calculate enthalpy changes in heating. Heating Curve For Sodium.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Heating Curve For Sodium a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two types of plots provide. heating curve for sodium chloride. phase diagrams (plots of pressure vs. Temperature) were correlated with heating curves (plots of temperature vs. the amount of energy needed to sublime 1 mol. Heating Curve For Sodium.
From brainly.in
The graph below represent the heating curve of substance X. i) What Heating Curve For Sodium the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. These two types of plots provide. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Temperature) were correlated with heating. Heating Curve For Sodium.
From www.doubtnut.com
The given graph shows the heating curve for a pure substance. The Heating Curve For Sodium a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Temperature) were correlated with heating curves (plots of temperature vs. phase diagrams (plots of pressure vs. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the amount. Heating Curve For Sodium.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve For Sodium phase diagrams (plots of pressure vs. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. These two types of plots provide.. Heating Curve For Sodium.
From www.jove.com
Heating and Cooling Curves JoVE Wordpress Development Heating Curve For Sodium Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. These two types of plots provide. Temperature) were correlated with heating curves (plots of temperature vs. heating curve for sodium chloride. How to calculate enthalpy changes in heating & cooling | crash chemistry a plot of the temperature versus. Heating Curve For Sodium.
From www.researchgate.net
The heating (a) and cooling (b) curves of neat PP, lactic casein (LAC Heating Curve For Sodium Temperature) were correlated with heating curves (plots of temperature vs. How to calculate enthalpy changes in heating & cooling | crash chemistry a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). heating curve for sodium chloride. Not all substances melt (or freeze) at 0 °c and boil. Heating Curve For Sodium.
From www.chegg.com
Solved Heating and Cooling Curve Heat Curve for C12 Heating Heating Curve For Sodium These two types of plots provide. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. Temperature) were correlated with heating curves (plots. Heating Curve For Sodium.
From www.showme.com
Cooling curve of sodium chloride Science ShowMe Heating Curve For Sodium How to calculate enthalpy changes in heating & cooling | crash chemistry phase diagrams (plots of pressure vs. heating curve for sodium chloride. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two types of plots provide. Not all substances melt (or freeze) at 0. Heating Curve For Sodium.
From fyodsdvxn.blob.core.windows.net
Heating And Cooling Curves Of Substances at Raymond Leftwich blog Heating Curve For Sodium How to calculate enthalpy changes in heating & cooling | crash chemistry These two types of plots provide. phase diagrams (plots of pressure vs. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Not all substances melt (or freeze) at. Heating Curve For Sodium.
From www.researchgate.net
4. Hydrochloric acid and sodium hydroxide neutralisation curves, [99 Heating Curve For Sodium phase diagrams (plots of pressure vs. How to calculate enthalpy changes in heating & cooling | crash chemistry Temperature) were correlated with heating curves (plots of temperature vs. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). heating curve for sodium chloride. These two types of. Heating Curve For Sodium.
From hxelkzbwv.blob.core.windows.net
Heating Curve Of Water Real Time at Joshua Oneal blog Heating Curve For Sodium Temperature) were correlated with heating curves (plots of temperature vs. These two types of plots provide. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. How to calculate enthalpy changes in heating & cooling | crash chemistry phase diagrams (plots of pressure vs. the amount of energy needed. Heating Curve For Sodium.
From www.showme.com
Cooling curve of sodium chloride (Priscillia & Chloe) Science ShowMe Heating Curve For Sodium a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two types of plots provide. Temperature) were correlated with heating curves (plots of temperature vs. heating curve for sodium chloride. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like. Heating Curve For Sodium.
From www.slideserve.com
PPT Heating Curves and Specific Heat PowerPoint Presentation, free Heating Curve For Sodium Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. These two types of plots provide. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Temperature) were correlated with heating curves (plots. Heating Curve For Sodium.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve For Sodium Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. These two types of plots provide. How to calculate enthalpy changes in heating. Heating Curve For Sodium.
From studylib.net
heating curve lab Heating Curve For Sodium How to calculate enthalpy changes in heating & cooling | crash chemistry heating curve for sodium chloride. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. a plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). These two. Heating Curve For Sodium.
From www.gauthmath.com
Solved 3) What is the melting point of sodium chloride in the graph Heating Curve For Sodium Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. a plot of the temperature versus the amount of heat added is. Heating Curve For Sodium.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve For Sodium Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. Temperature) were correlated with heating curves (plots of temperature vs. phase diagrams (plots of pressure vs. heating curve for sodium chloride. How to calculate enthalpy changes in heating & cooling | crash chemistry These two types of plots provide.. Heating Curve For Sodium.
From www.slideserve.com
PPT OB Practice phase concepts cooling and heating curves, phase Heating Curve For Sodium These two types of plots provide. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. heating curve for sodium chloride. Not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. How. Heating Curve For Sodium.
From curiophysics.com
Heating Curve » Curio Physics Heating Curve For Sodium These two types of plots provide. the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. Temperature) were correlated with heating curves (plots of temperature vs. phase diagrams (plots of pressure vs. Not all substances melt (or freeze) at 0 °c. Heating Curve For Sodium.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve For Sodium the amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the enthalpies of fusion. These two types of plots provide. heating curve for sodium chloride. phase diagrams (plots of pressure vs. How to calculate enthalpy changes in heating & cooling | crash chemistry . Heating Curve For Sodium.