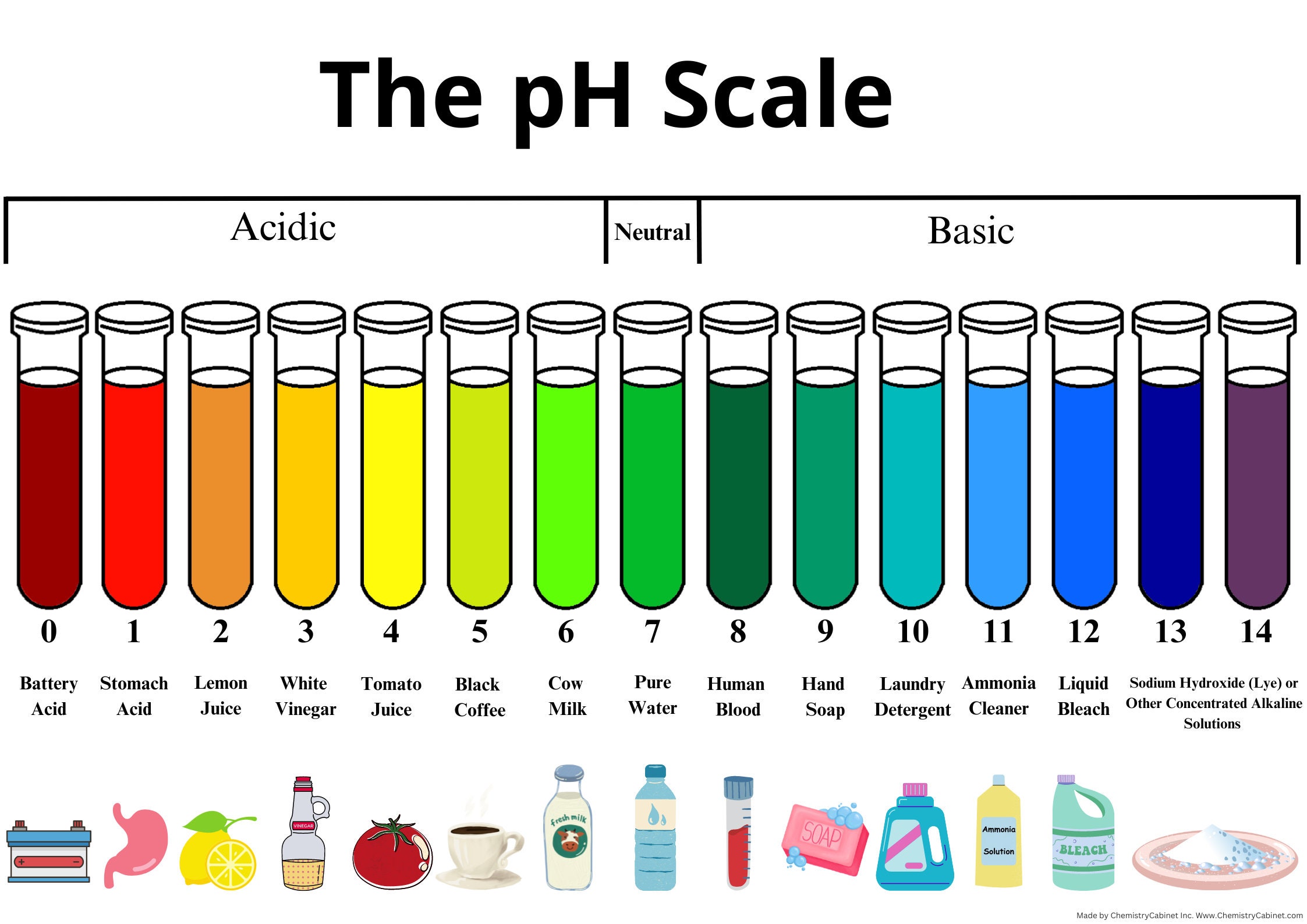
Buffers And Ph Lab . Create buffer solutions and test the effects of adding acid. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. To create and study the properties of buffer solutions. Ph buffers the basic idea: It is able to neutralize small amounts of added acid or base, thus. To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0. The ph scale ranges from 0 to 14, with a ph of 7 being. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A solution with a high number of hydroxide ions is basic and has a high ph value. Using indicators to measure ph. In this part of the experiment you will use five indicators to determine the ph. Thus, they are considerably more. Accurately measure the ph of solutions using ph indicator strips and a ph meter.
from www.etsy.com
A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. The ph scale ranges from 0 to 14, with a ph of 7 being. Using indicators to measure ph. A weak acid and it’s conjugate base in equilibrium: This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0. Create buffer solutions and test the effects of adding acid. Ph buffers the basic idea: A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.
Ph Scale Chart Print PDF Download Chemistry for Classroom Etsy Ireland
Buffers And Ph Lab Accurately measure the ph of solutions using ph indicator strips and a ph meter. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. A weak acid and it’s conjugate base in equilibrium: In this part of the experiment you will use five indicators to determine the ph. A solution with a high number of hydroxide ions is basic and has a high ph value. Thus, they are considerably more. Using indicators to measure ph. Create buffer solutions and test the effects of adding acid. Ph buffers the basic idea: It is able to neutralize small amounts of added acid or base, thus. Accurately measure the ph of solutions using ph indicator strips and a ph meter. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ph scale ranges from 0 to 14, with a ph of 7 being. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. To create and study the properties of buffer solutions.
From www.chegg.com
Solved Lab 3 pH and Buffers Discussion A. The pH scale and Buffers And Ph Lab A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A weak acid and it’s conjugate base in equilibrium: Accurately measure the ph of solutions using ph indicator strips and a ph meter. A buffer is a solution that can resist ph change upon the addition of an. Buffers And Ph Lab.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffers And Ph Lab The ph scale ranges from 0 to 14, with a ph of 7 being. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In this part of the experiment you will use five indicators to determine the ph. Strong laboratory acids typically have ph values less than. Buffers And Ph Lab.
From www.vrogue.co
Solved Ph Report Sheet Acids Bases Ph And Buffers A C vrogue.co Buffers And Ph Lab In this part of the experiment you will use five indicators to determine the ph. Ph buffers the basic idea: To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0. A weak acid. Buffers And Ph Lab.
From www.chegg.com
Solved Experiment VII Buffers Lab Report ( 49 pts) I. Buffers And Ph Lab Using indicators to measure ph. To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0. In this part of the experiment you will use five indicators to determine the ph. This characteristic makes. Buffers And Ph Lab.
From www.studocu.com
Post Lab 1 Buffers and pH Introduction Buffers are an essential Buffers And Ph Lab Accurately measure the ph of solutions using ph indicator strips and a ph meter. Using indicators to measure ph. Create buffer solutions and test the effects of adding acid. In this part of the experiment you will use five indicators to determine the ph. To understand how well a buffer protects against changes in ph, consider the effect of adding.01. Buffers And Ph Lab.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Buffers And Ph Lab Ph buffers the basic idea: Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. Accurately measure the ph of solutions using ph indicator strips and a ph meter. In this part of the experiment you will use five indicators to determine the ph. To understand. Buffers And Ph Lab.
From www.chegg.com
Solved Acids Bases, pH, and Buffers Lab Information 21/ hr Buffers And Ph Lab Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. Thus, they are considerably more. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In this part of the experiment you will use five indicators to. Buffers And Ph Lab.
From www.chegg.com
Acids, Bases, Buffers and pH Postlaboratory Buffers And Ph Lab Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. In this part of the experiment you will use five indicators to determine the ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ph. Buffers And Ph Lab.
From www.youtube.com
Buffers and pH Biology YouTube Buffers And Ph Lab Ph buffers the basic idea: A weak acid and it’s conjugate base in equilibrium: The ph scale ranges from 0 to 14, with a ph of 7 being. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. Using indicators to measure ph. A solution with. Buffers And Ph Lab.
From www.fishersci.fi
Buffer Solution, pH 7.00, ChemLab Buffers Buffers and Solutions Buffers And Ph Lab A weak acid and it’s conjugate base in equilibrium: A solution with a high number of hydroxide ions is basic and has a high ph value. The ph scale ranges from 0 to 14, with a ph of 7 being. Accurately measure the ph of solutions using ph indicator strips and a ph meter. To understand how well a buffer. Buffers And Ph Lab.
From myans.bhantedhammika.net
Hydrolysis Of Salts Lab Answers Buffers And Ph Lab Thus, they are considerably more. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Accurately measure the ph of solutions using ph indicator strips and a ph meter. In this part of the experiment you will use five indicators to determine the ph. To create and study the properties of. Buffers And Ph Lab.
From www.chegg.com
Solved Acids, Bases, Buffers and p Postlaboratory Buffers And Ph Lab Using indicators to measure ph. Thus, they are considerably more. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical. Buffers And Ph Lab.
From www.chegg.com
Solved Exp 25. pH MeasurementsBuffers and Their Properties. Buffers And Ph Lab A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Ph buffers the basic idea: This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically. Buffers And Ph Lab.
From www.brainkart.com
Buffers Buffers And Ph Lab Accurately measure the ph of solutions using ph indicator strips and a ph meter. To create and study the properties of buffer solutions. It is able to neutralize small amounts of added acid or base, thus. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that maintains the stability. Buffers And Ph Lab.
From www.studocu.com
P H Measurement 313 lab report pH Measurement and its Applications Buffers And Ph Lab In this part of the experiment you will use five indicators to determine the ph. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or. Buffers And Ph Lab.
From www.coursehero.com
[Solved] Buffers And Ph Lab Accurately measure the ph of solutions using ph indicator strips and a ph meter. Thus, they are considerably more. A solution with a high number of hydroxide ions is basic and has a high ph value. Using indicators to measure ph. The ph scale ranges from 0 to 14, with a ph of 7 being. This characteristic makes buffers important. Buffers And Ph Lab.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Buffers And Ph Lab This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Accurately measure the ph of solutions using ph indicator strips and a ph meter. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To understand how well a buffer protects against changes in ph, consider. Buffers And Ph Lab.
From www.studocu.com
Acids, Bases, and p H Buffers SA answers Drylab Acids, Bases, and Buffers And Ph Lab A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water. Buffers And Ph Lab.
From zanenewswaller.blogspot.com
Why Buffer Solution Is Used in Edta Titration Buffers And Ph Lab A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. To create and study the properties of buffer solutions. The ph scale ranges from 0 to 14, with a ph of 7 being. Using indicators to measure ph. A weak acid and it’s conjugate base in equilibrium: Ph. Buffers And Ph Lab.
From www.etsy.com
Ph Scale Chart Print PDF Download Chemistry for Classroom Etsy Ireland Buffers And Ph Lab A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A weak acid and it’s conjugate base in equilibrium: To create and study the properties of buffer solutions. A solution with a high number of hydroxide ions is basic and has a high ph value. A buffer is. Buffers And Ph Lab.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Buffers And Ph Lab Accurately measure the ph of solutions using ph indicator strips and a ph meter. To create and study the properties of buffer solutions. The ph scale ranges from 0 to 14, with a ph of 7 being. Thus, they are considerably more. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. Buffers And Ph Lab.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Buffers And Ph Lab To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0. Thus, they are considerably more. Accurately measure the ph of solutions using ph indicator strips and a ph meter. The ph scale ranges. Buffers And Ph Lab.
From acidsandbasesfordummieschem.weebly.com
Buffers Acid and Bases for Dummies! Buffers And Ph Lab A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Accurately measure the ph of solutions using ph indicator strips and a ph meter. To create and study the properties of buffer solutions. Ph buffers the basic idea: A solution with a high number of hydroxide ions is. Buffers And Ph Lab.
From www.numerade.com
SOLVED REPORT SHEET LAB Acids, Bases, pH, and Buffers Reference Buffers And Ph Lab To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0. A solution with a high number of hydroxide ions is basic and has a high ph value. Ph buffers the basic idea: A. Buffers And Ph Lab.
From www.indiamart.com
PH Buffer Solution, Grade Standard Reagent Grade, for Laboratory, Rs Buffers And Ph Lab A weak acid and it’s conjugate base in equilibrium: To create and study the properties of buffer solutions. Thus, they are considerably more. To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0.. Buffers And Ph Lab.
From www.mcguffmedical.com
pH Calibrating Buffer Solution, pH 4.01, 475mL, Each McGuff Medical Buffers And Ph Lab Using indicators to measure ph. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The ph scale ranges from 0 to 14, with a ph of 7 being. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Ph buffers the basic. Buffers And Ph Lab.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Buffers And Ph Lab Using indicators to measure ph. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. A weak acid and it’s conjugate base in equilibrium: To create and study the properties of buffer solutions. The ph scale ranges from 0 to 14, with a ph of 7. Buffers And Ph Lab.
From www.indiamart.com
Ww 00654xx Oakton pH Buffer Solution 500 mL, Grade Standard Buffers And Ph Lab Accurately measure the ph of solutions using ph indicator strips and a ph meter. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A weak acid and it’s conjugate base in equilibrium: To create and study the. Buffers And Ph Lab.
From www.youtube.com
Acids, Bases, pH, Buffers YouTube Buffers And Ph Lab A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Ph buffers the basic idea: Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values. Buffers And Ph Lab.
From www.scribd.com
pH and Buffers Acid Buffer Solution Buffers And Ph Lab It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In this part of the experiment you will use five indicators to determine the ph. To understand how well a buffer protects against changes in ph, consider the. Buffers And Ph Lab.
From animalia-life.club
Phosphate Buffer System Equation Buffers And Ph Lab In this part of the experiment you will use five indicators to determine the ph. Ph buffers the basic idea: The ph scale ranges from 0 to 14, with a ph of 7 being. A weak acid and it’s conjugate base in equilibrium: Accurately measure the ph of solutions using ph indicator strips and a ph meter. It is able. Buffers And Ph Lab.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffers And Ph Lab Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. It is able to neutralize small amounts of added acid or base, thus. Create buffer solutions and test the effects of adding acid. A buffer is a solution that can resist ph change upon the addition. Buffers And Ph Lab.
From docslib.org
Ph and Buffers Laboratory DocsLib Buffers And Ph Lab Accurately measure the ph of solutions using ph indicator strips and a ph meter. Strong laboratory acids typically have ph values less than 0 (negative ph values) and strong laboratory bases typically have ph values greater than 14. To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter. Buffers And Ph Lab.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Buffers And Ph Lab To create and study the properties of buffer solutions. Using indicators to measure ph. It is able to neutralize small amounts of added acid or base, thus. Thus, they are considerably more. Create buffer solutions and test the effects of adding acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic. Buffers And Ph Lab.
From www.coursehero.com
[Solved] Buffers And Ph Lab The ph scale ranges from 0 to 14, with a ph of 7 being. To understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0. To create and study the properties of buffer solutions.. Buffers And Ph Lab.