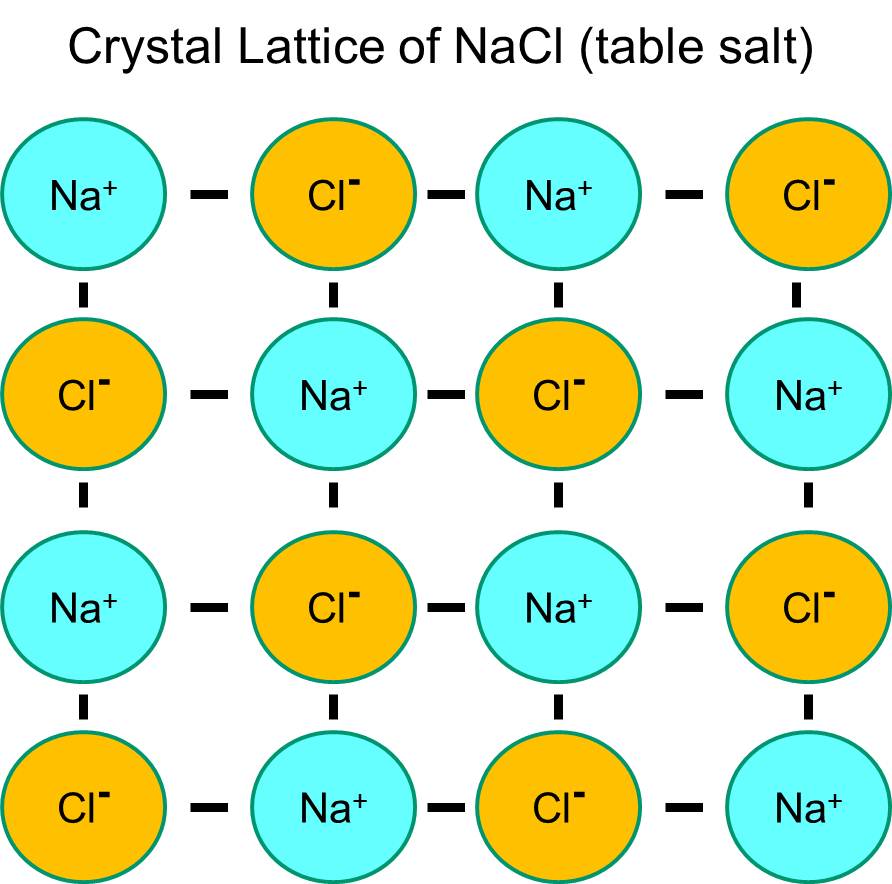
Chlorine Positive Ion . Compounds formed from positive and negative ions are ionic compounds. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. Individual atoms can gain or lose electrons. On the left, the chlorine atom has 17 electrons. Neutral chlorine atom on left has. 93 rows there are four ways to find the charge of an element: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. The usual charge of an element is common to its group. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. On the right, the chloride ion has 18 electrons and has a 1− charge.
from sphweb.bumc.bu.edu
93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. The usual charge of an element is common to its group. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. 93 rows there are four ways to find the charge of an element: Compounds formed from positive and negative ions are ionic compounds. On the left, the chlorine atom has 17 electrons. Neutral chlorine atom on left has. On the right, the chloride ion has 18 electrons and has a 1− charge. Individual atoms can gain or lose electrons. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable.
Chemical Elements Atoms
Chlorine Positive Ion On the right, the chloride ion has 18 electrons and has a 1− charge. The usual charge of an element is common to its group. Neutral chlorine atom on left has. On the left, the chlorine atom has 17 electrons. On the right, the chloride ion has 18 electrons and has a 1− charge. Compounds formed from positive and negative ions are ionic compounds. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. Individual atoms can gain or lose electrons. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. 93 rows there are four ways to find the charge of an element:
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine Positive Ion Neutral chlorine atom on left has. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. Compounds formed from positive and negative ions are ionic compounds. An ion is formed when either one or more electrons are. Chlorine Positive Ion.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Chlorine Positive Ion Individual atoms can gain or lose electrons. 93 rows there are four ways to find the charge of an element: The usual charge of an element is common to its group. On the left, the chlorine atom has 17 electrons. On the right, the chloride ion has 18 electrons and has a 1− charge. Compounds formed from positive and negative. Chlorine Positive Ion.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Positive Ion Individual atoms can gain or lose electrons. Compounds formed from positive and negative ions are ionic compounds. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. An atom of sodium (na) donates one of its electrons to. Chlorine Positive Ion.
From www.dreamstime.com
Diagram Representation of the Element Chlorine Stock Illustration Chlorine Positive Ion 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. 93 rows there are four ways to find the charge of an element: Individual atoms can gain or lose electrons. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl). Chlorine Positive Ion.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Chlorine Positive Ion An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. Individual atoms can gain or lose electrons. 93 rows there are four ways to find the charge of an element: An ion is formed when either one. Chlorine Positive Ion.
From brainly.in
draw atomic structure of chlorine Brainly.in Chlorine Positive Ion Neutral chlorine atom on left has. Individual atoms can gain or lose electrons. On the right, the chloride ion has 18 electrons and has a 1− charge. Compounds formed from positive and negative ions are ionic compounds. The usual charge of an element is common to its group. An ion is formed when either one or more electrons are removed. Chlorine Positive Ion.
From www.slideserve.com
PPT Ionic Bonds PowerPoint Presentation, free download ID6301347 Chlorine Positive Ion Compounds formed from positive and negative ions are ionic compounds. Individual atoms can gain or lose electrons. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. On the right, the chloride ion has 18 electrons and has. Chlorine Positive Ion.
From www.numerade.com
SOLVED Which two particles can form an ionic bond with each other? A Chlorine Positive Ion On the right, the chloride ion has 18 electrons and has a 1− charge. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. On the left, the chlorine atom has 17 electrons. The usual charge of an element is common to its group. Compounds formed. Chlorine Positive Ion.
From www.slideserve.com
PPT Ionic Bonds (the attraction between negative ions & positive ions Chlorine Positive Ion An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach. Chlorine Positive Ion.
From www.dreamstime.com
Anions and Cations for Example Sodium and Chlorine Atoms. Stock Vector Chlorine Positive Ion On the right, the chloride ion has 18 electrons and has a 1− charge. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation). Chlorine Positive Ion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Chlorine Positive Ion Individual atoms can gain or lose electrons. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. 93 rows there are four ways to find the charge of an element: Compounds formed from positive and negative ions. Chlorine Positive Ion.
From gardenandplate.com
Molecules Chlorine Positive Ion On the right, the chloride ion has 18 electrons and has a 1− charge. Individual atoms can gain or lose electrons. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. 93 rows there are four ways. Chlorine Positive Ion.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Positive Ion On the right, the chloride ion has 18 electrons and has a 1− charge. On the left, the chlorine atom has 17 electrons. Compounds formed from positive and negative ions are ionic compounds. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach. Chlorine Positive Ion.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 di Bonding and the Chlorine Positive Ion Neutral chlorine atom on left has. The usual charge of an element is common to its group. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. On the right, the chloride ion has 18 electrons and. Chlorine Positive Ion.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Chlorine Positive Ion 93 rows there are four ways to find the charge of an element: On the right, the chloride ion has 18 electrons and has a 1− charge. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable.. Chlorine Positive Ion.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Positive Ion An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. 93 rows there are four ways to find the charge of an element: Neutral chlorine atom on left has. Compounds formed from positive and negative ions are ionic. Chlorine Positive Ion.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other Chlorine Positive Ion On the left, the chlorine atom has 17 electrons. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. An ion is formed when either one or more electrons are removed from a neutral atom to form. Chlorine Positive Ion.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Positive Ion 93 rows there are four ways to find the charge of an element: Compounds formed from positive and negative ions are ionic compounds. The usual charge of an element is common to its group. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons. Chlorine Positive Ion.
From www.slideserve.com
PPT Neurons and Synapses PowerPoint Presentation, free download ID Chlorine Positive Ion 93 rows there are four ways to find the charge of an element: 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. On the right, the chloride ion has 18 electrons and has a 1− charge. On the left, the chlorine atom has 17 electrons.. Chlorine Positive Ion.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Chlorine Positive Ion On the left, the chlorine atom has 17 electrons. Compounds formed from positive and negative ions are ionic compounds. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. The usual charge of an element is common. Chlorine Positive Ion.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Chlorine Positive Ion An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. Compounds formed from positive and negative ions are ionic compounds. 93 rows there are four ways to find the charge of an element: On the right, the chloride. Chlorine Positive Ion.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Chlorine Positive Ion An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. Neutral chlorine atom on left has. 93 rows there are four ways to find the charge of an element: The usual charge of an element is common. Chlorine Positive Ion.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Chlorine Positive Ion 93 rows there are four ways to find the charge of an element: On the left, the chlorine atom has 17 electrons. On the right, the chloride ion has 18 electrons and has a 1− charge. Compounds formed from positive and negative ions are ionic compounds. Individual atoms can gain or lose electrons. An atom of sodium (na) donates one. Chlorine Positive Ion.
From basichemistry.blogspot.com
Basic Chemistry October 2012 Chlorine Positive Ion An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. Individual atoms can gain or lose electrons. Neutral chlorine atom on left has. An atom of sodium (na) donates one of its electrons to an atom of chlorine. Chlorine Positive Ion.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Positive Ion The usual charge of an element is common to its group. 93 rows there are four ways to find the charge of an element: Neutral chlorine atom on left has. On the left, the chlorine atom has 17 electrons. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged. Chlorine Positive Ion.
From www.numerade.com
SOLVED [Choose ] Negatively charged ion of Carbon Isotope of Chlorine Chlorine Positive Ion On the right, the chloride ion has 18 electrons and has a 1− charge. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. The usual charge of an element is common to its group. 93 rows. Chlorine Positive Ion.
From www.onlinechemistrytutor.net
chlorine001 Online Chemistry Tutor Chlorine Positive Ion On the left, the chlorine atom has 17 electrons. The usual charge of an element is common to its group. On the right, the chloride ion has 18 electrons and has a 1− charge. Neutral chlorine atom on left has. Individual atoms can gain or lose electrons. An atom of sodium (na) donates one of its electrons to an atom. Chlorine Positive Ion.
From design.udlvirtual.edu.pe
What Does It Mean When An Ion Has A Positive Charge Design Talk Chlorine Positive Ion On the left, the chlorine atom has 17 electrons. Compounds formed from positive and negative ions are ionic compounds. The usual charge of an element is common to its group. Individual atoms can gain or lose electrons. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. Chlorine Positive Ion.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Positive Ion An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. Individual atoms can gain or lose electrons. The usual charge of an element is common to its group. Neutral chlorine atom on left has. Compounds formed from positive. Chlorine Positive Ion.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Positive Ion Individual atoms can gain or lose electrons. Compounds formed from positive and negative ions are ionic compounds. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. On the left, the chlorine atom has 17 electrons. An atom of sodium (na) donates one of its electrons. Chlorine Positive Ion.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Positive Ion An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative. Chlorine Positive Ion.
From exowfmlsz.blob.core.windows.net
Chlorine Ion Charge Formula at Angela Orlando blog Chlorine Positive Ion Neutral chlorine atom on left has. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na+) and negative ion (cl−) form a stable. On the right, the chloride ion has 18 electrons and has a 1− charge. Individual atoms can gain or lose electrons.. Chlorine Positive Ion.
From www.britannica.com
halogen Facts, Definition, Properties, & Uses Britannica Chlorine Positive Ion 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Neutral chlorine atom on left has. 93 rows there are four ways to find the charge of an element: Compounds formed from positive and negative ions are ionic compounds. On the right, the chloride ion has. Chlorine Positive Ion.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Chlorine Positive Ion 93 rows there are four ways to find the charge of an element: The usual charge of an element is common to its group. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. An ion is formed when either one or more electrons are removed. Chlorine Positive Ion.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Positive Ion The usual charge of an element is common to its group. 93 rows there are four ways to find the charge of an element: On the right, the chloride ion has 18 electrons and has a 1− charge. Neutral chlorine atom on left has. An ion is formed when either one or more electrons are removed from a neutral atom. Chlorine Positive Ion.