Which Is The Smallest Transition Metal . The most common definition of a transition metal is the one accepted by the iupac. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Iupac defines transition elements as an. Which transition metal has the most number of. Most of the elements of the first. When heated in a flame, the element indium emits. Alkali metals and alkaline earth metals)? Based on your observations, in which metal did the smallest electronic transitions occur? These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common.
from utedzz.blogspot.com
Most of the elements of the first. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iupac defines transition elements as an. Alkali metals and alkaline earth metals)? Which transition metal has the most number of. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Based on your observations, in which metal did the smallest electronic transitions occur? The most common definition of a transition metal is the one accepted by the iupac. When heated in a flame, the element indium emits.
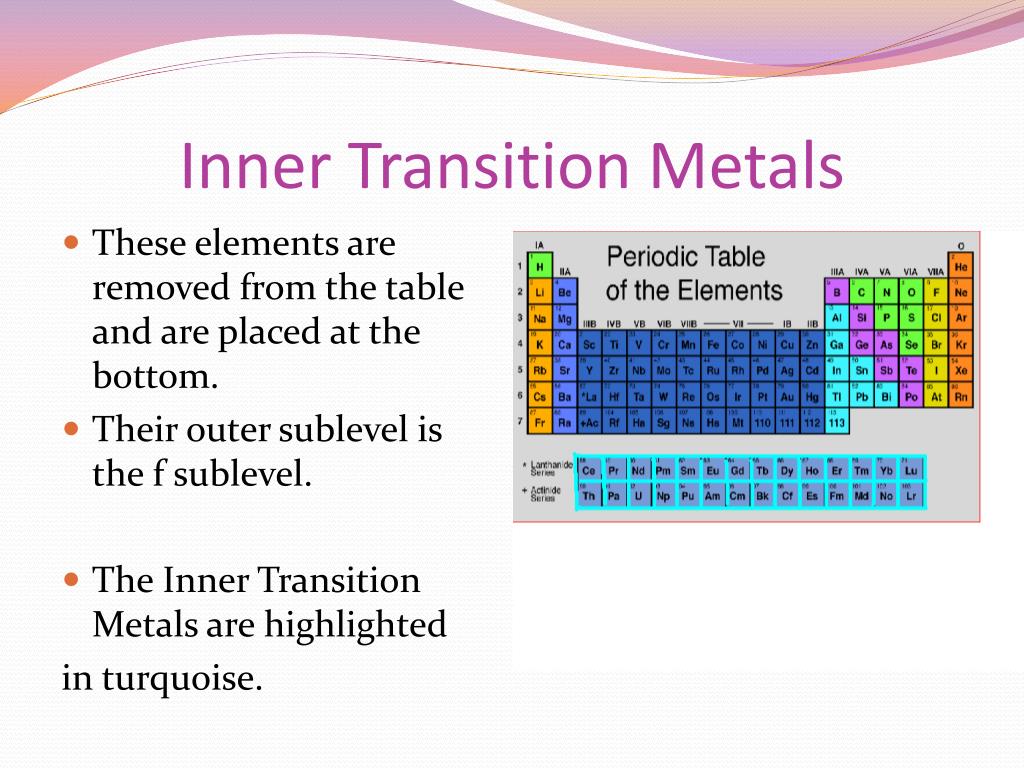
Periodic Table Of Elements Inner Transition Metals Periodic Table
Which Is The Smallest Transition Metal Alkali metals and alkaline earth metals)? Most of the elements of the first. Iupac defines transition elements as an. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Based on your observations, in which metal did the smallest electronic transitions occur? Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Alkali metals and alkaline earth metals)? These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. When heated in a flame, the element indium emits. The most common definition of a transition metal is the one accepted by the iupac. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Which transition metal has the most number of.
From byjus.com
As the oxidation number of a transition metal in transition metal oxide Which Is The Smallest Transition Metal Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Alkali metals and alkaline earth metals)? The most common definition of a transition metal is the one accepted by the iupac. Which transition metal has the most number of. These elements are called transition metals because electrons in their. Which Is The Smallest Transition Metal.
From www.chegg.com
Solved Which of the following transition metals would be Which Is The Smallest Transition Metal Transition elements (also known as transition metals) are elements that have partially filled d orbitals. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Based on your observations, in which metal did the smallest electronic transitions occur? Which transition metal has the most number of. Alkali metals and. Which Is The Smallest Transition Metal.
From www.slideserve.com
PPT THE TRANSITION METALS PowerPoint Presentation, free download ID Which Is The Smallest Transition Metal Which transition metal has the most number of. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Iupac defines transition elements as an. Based on your observations, in which metal did the smallest. Which Is The Smallest Transition Metal.
From www.thesciencehive.co.uk
Transition Elements* — the science sauce Which Is The Smallest Transition Metal Most of the elements of the first. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. The most common definition of a transition metal is the one accepted by the iupac. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Transition. Which Is The Smallest Transition Metal.
From ar.inspiredpencil.com
Transition Metals Which Is The Smallest Transition Metal The most common definition of a transition metal is the one accepted by the iupac. Based on your observations, in which metal did the smallest electronic transitions occur? Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Most of the elements of the first. Alkali metals and alkaline earth metals)? These elements are called. Which Is The Smallest Transition Metal.
From sciencenotes.org
Transition Metals Definition, List and Properties Which Is The Smallest Transition Metal Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Most of the elements of the first. When heated in a flame, the element indium emits. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Based on your observations, in which metal did the smallest electronic transitions occur?. Which Is The Smallest Transition Metal.
From www.haikudeck.com
Transition Metals by Sarena Kinkel Which Is The Smallest Transition Metal Most of the elements of the first. Alkali metals and alkaline earth metals)? When heated in a flame, the element indium emits. The most common definition of a transition metal is the one accepted by the iupac. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iron is known to form oxidation states. Which Is The Smallest Transition Metal.
From www.pinterest.com
In this section i am going to talk about Chemical Families. The red Which Is The Smallest Transition Metal Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iupac defines transition elements as an. Most of the elements of the first. Based on your observations, in which metal did the smallest. Which Is The Smallest Transition Metal.
From www.nagwa.com
Question Video Determining Which Alkali Metal Has the Smallest Atomic Which Is The Smallest Transition Metal Which transition metal has the most number of. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Most of the elements of the first. The most common definition of a transition metal is the one accepted by the iupac. Transition elements (also known as transition metals) are elements. Which Is The Smallest Transition Metal.
From www.nagwa.com
Question Video Identifying the Transition Metal with the Lowest Which Is The Smallest Transition Metal Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Iupac defines transition elements as an. Which transition metal has the most number of. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. These elements are called transition metals because electrons in their. Which Is The Smallest Transition Metal.
From www.vedantu.com
Uses of Transition Metals Learn Important Terms and Concepts Which Is The Smallest Transition Metal These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Which transition metal has the most number of. Transition elements (also known as transition metals) are elements that. Which Is The Smallest Transition Metal.
From www.bartleby.com
Answered Which of the following transition… bartleby Which Is The Smallest Transition Metal The most common definition of a transition metal is the one accepted by the iupac. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Iupac defines transition elements as an. Based on your observations, in which metal did the smallest electronic transitions occur? Most of the elements of. Which Is The Smallest Transition Metal.
From utedzz.blogspot.com
Periodic Table Of Elements Inner Transition Metals Periodic Table Which Is The Smallest Transition Metal Most of the elements of the first. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iupac. Which Is The Smallest Transition Metal.
From studymind.co.uk
The Transition Metals (GCSE Chemistry) Study Mind Which Is The Smallest Transition Metal Alkali metals and alkaline earth metals)? The most common definition of a transition metal is the one accepted by the iupac. Which transition metal has the most number of. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Based on your observations, in which metal did the smallest. Which Is The Smallest Transition Metal.
From www.youtube.com
Properties of the TRANSITION METALS Periodic Table YouTube Which Is The Smallest Transition Metal Most of the elements of the first. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Which transition metal has the most number of. When heated in a flame, the element indium emits. Based on your observations, in which metal did the smallest electronic transitions occur? These elements. Which Is The Smallest Transition Metal.
From socratic.org
For a given Period, which is the smallest atom? Socratic Which Is The Smallest Transition Metal Iupac defines transition elements as an. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Alkali metals and alkaline earth metals)? Transition elements (also known as transition. Which Is The Smallest Transition Metal.
From chem.libretexts.org
8.6 Group 13 (and a note on the posttransition metals) Chemistry Which Is The Smallest Transition Metal Iupac defines transition elements as an. Which transition metal has the most number of. Most of the elements of the first. Alkali metals and alkaline earth metals)? Transition elements (also known as transition metals) are elements that have partially filled d orbitals. The most common definition of a transition metal is the one accepted by the iupac. Based on your. Which Is The Smallest Transition Metal.
From www.priyamstudycentre.com
Transition Metals Elements, Definition, List, Properties Which Is The Smallest Transition Metal The most common definition of a transition metal is the one accepted by the iupac. Iupac defines transition elements as an. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being. Which Is The Smallest Transition Metal.
From www.youtube.com
The Transition Metals YouTube Which Is The Smallest Transition Metal Iupac defines transition elements as an. When heated in a flame, the element indium emits. Based on your observations, in which metal did the smallest electronic transitions occur? Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. The most common definition of a transition metal is the one. Which Is The Smallest Transition Metal.
From ar.inspiredpencil.com
Periodic Table Of Elements Transition Metals Which Is The Smallest Transition Metal When heated in a flame, the element indium emits. Alkali metals and alkaline earth metals)? Which transition metal has the most number of. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Most of the elements of the first. Iupac defines transition elements as an. The most common definition of a transition metal. Which Is The Smallest Transition Metal.
From saylordotorg.github.io
General Trends among the Transition Metals Which Is The Smallest Transition Metal Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Alkali metals and alkaline earth metals)? Based on your observations, in which metal did the smallest electronic transitions occur? Transition elements (also known. Which Is The Smallest Transition Metal.
From socratic.org
Which are the smallest and largest atoms? Socratic Which Is The Smallest Transition Metal The most common definition of a transition metal is the one accepted by the iupac. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Which transition metal has the most number of. Iupac defines transition elements as an. These elements are called transition metals because electrons in their. Which Is The Smallest Transition Metal.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica Which Is The Smallest Transition Metal Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Most of the elements of the first. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Iupac. Which Is The Smallest Transition Metal.
From byjus.com
Which group contains the inner transition elements? Which Is The Smallest Transition Metal Iupac defines transition elements as an. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Based on your observations, in which metal did the smallest electronic transitions occur? Alkali metals and alkaline earth. Which Is The Smallest Transition Metal.
From www.slideserve.com
PPT Transition Metals PowerPoint Presentation, free download ID2275684 Which Is The Smallest Transition Metal Based on your observations, in which metal did the smallest electronic transitions occur? Why do transition metals have a greater number of oxidation states than main group metals (i.e. Which transition metal has the most number of. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Alkali metals and alkaline earth metals)? These elements. Which Is The Smallest Transition Metal.
From sorenancebentley.blogspot.com
Copper is a Metal or Nonmetal SorenanceBentley Which Is The Smallest Transition Metal Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Iupac defines transition elements as an. Which transition metal has the most number of. Alkali metals and alkaline earth metals)? When heated in a flame, the element indium emits. Why do transition metals have a greater number of oxidation. Which Is The Smallest Transition Metal.
From chem.libretexts.org
General Trends among the Transition Metals Chemistry LibreTexts Which Is The Smallest Transition Metal Transition elements (also known as transition metals) are elements that have partially filled d orbitals. The most common definition of a transition metal is the one accepted by the iupac. Alkali metals and alkaline earth metals)? Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Why do transition. Which Is The Smallest Transition Metal.
From commons.wikimedia.org
FileTransition metals in the periodic table.png Wikimedia Commons Which Is The Smallest Transition Metal Based on your observations, in which metal did the smallest electronic transitions occur? When heated in a flame, the element indium emits. Iupac defines transition elements as an. Most of the elements of the first. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Why do transition metals have a greater number of oxidation. Which Is The Smallest Transition Metal.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation Which Is The Smallest Transition Metal Which transition metal has the most number of. Transition elements (also known as transition metals) are elements that have partially filled d orbitals. The most common definition of a transition metal is the one accepted by the iupac. Based on your observations, in which metal did the smallest electronic transitions occur? Alkali metals and alkaline earth metals)? When heated in. Which Is The Smallest Transition Metal.
From cabinet.matttroy.net
Periodic Table Charges Transition Metals Matttroy Which Is The Smallest Transition Metal Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii) being the most common. Which transition metal has the most number of.. Which Is The Smallest Transition Metal.
From brokeasshome.com
Where Is Transition Metals Located On The Periodic Table Which Is The Smallest Transition Metal Based on your observations, in which metal did the smallest electronic transitions occur? Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Most of the elements of the first. Alkali metals and alkaline earth metals)? These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d. Which Is The Smallest Transition Metal.
From period-faqs.com
Where Are The Transition Metals Located On The Periodic Table Which Is The Smallest Transition Metal Transition elements (also known as transition metals) are elements that have partially filled d orbitals. Most of the elements of the first. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Alkali metals and alkaline earth metals)? The most common definition of a transition metal is the one. Which Is The Smallest Transition Metal.
From utedzz.blogspot.com
Periodic Table Inner Transition Metals Periodic Table Timeline Which Is The Smallest Transition Metal Iupac defines transition elements as an. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Why do transition metals have a greater number of oxidation states than main group metals (i.e. Iron is known to form oxidation states from 2+ to 6+, with iron (ii) and iron (iii). Which Is The Smallest Transition Metal.
From ar.inspiredpencil.com
Periodic Table With Transition Metals Which Is The Smallest Transition Metal Iupac defines transition elements as an. Alkali metals and alkaline earth metals)? Which transition metal has the most number of. The most common definition of a transition metal is the one accepted by the iupac. When heated in a flame, the element indium emits. These elements are called transition metals because electrons in their atoms transition to fill the d. Which Is The Smallest Transition Metal.
From app.jove.com
Transition Metals Electron Configurations and Properties Concept Which Is The Smallest Transition Metal When heated in a flame, the element indium emits. Iupac defines transition elements as an. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Based on your observations, in which metal did the smallest electronic transitions occur? The most common definition of a transition metal is the one. Which Is The Smallest Transition Metal.