Cold Pack Chemical Reaction Equation . This quick and easy procedure will. making an instant cold pack introduction ever used a cold pack on an injury? In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. in an endothermic reaction, a substance takes heat from its surroundings. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. So how does it work? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. The sodium thiosulfate needs energy to.
from classfullkatmandu.z19.web.core.windows.net
In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. making an instant cold pack introduction ever used a cold pack on an injury? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. This quick and easy procedure will. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. in an endothermic reaction, a substance takes heat from its surroundings. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. The sodium thiosulfate needs energy to. So how does it work?
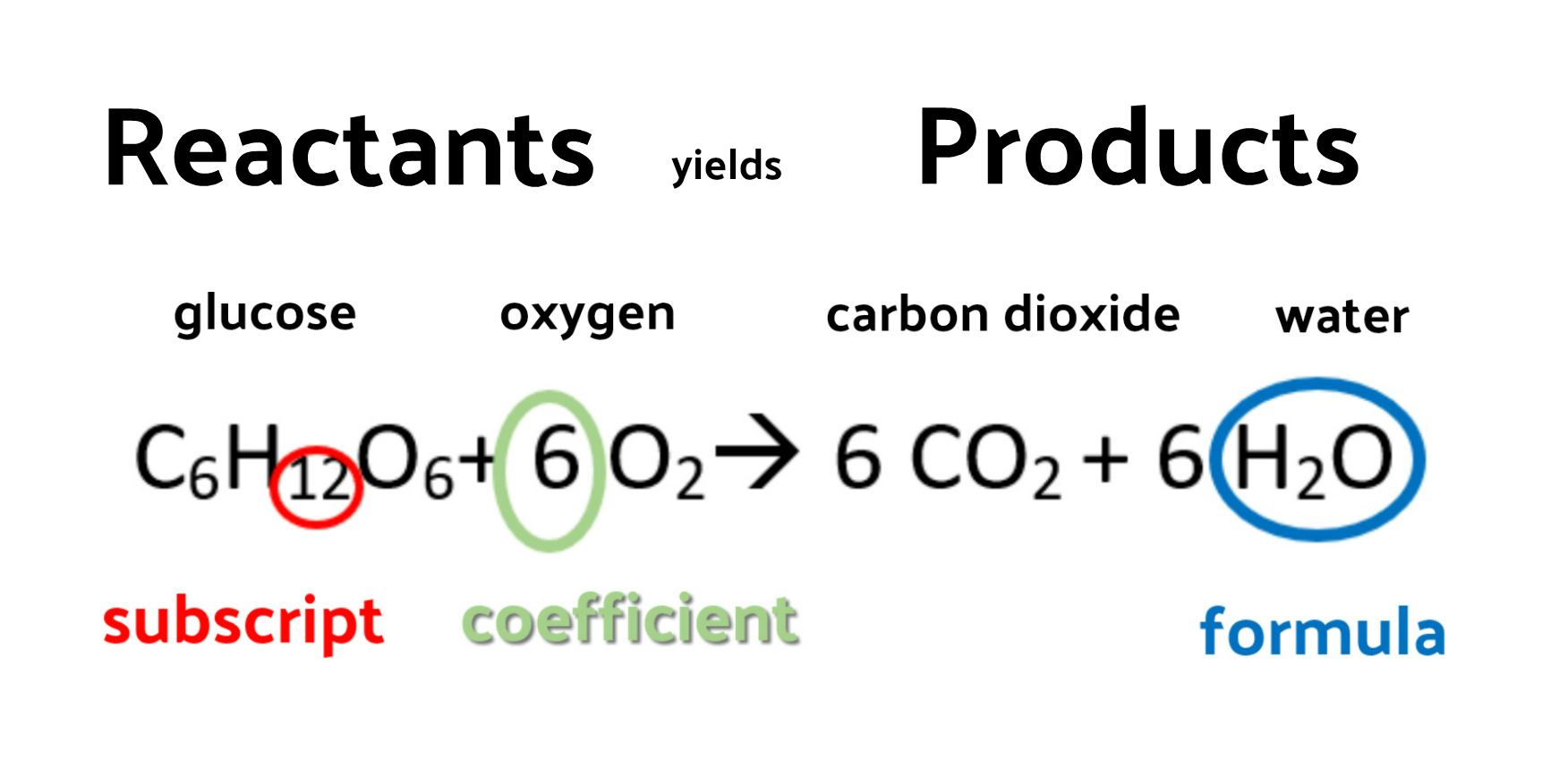
Chemical Reactions And Equation Notes
Cold Pack Chemical Reaction Equation So how does it work? making an instant cold pack introduction ever used a cold pack on an injury? So how does it work? A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. The sodium thiosulfate needs energy to. This quick and easy procedure will. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. in an endothermic reaction, a substance takes heat from its surroundings. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack.
From www.youtube.com
The chemistry of cold packs John Pollard YouTube Cold Pack Chemical Reaction Equation So how does it work? Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. This quick and easy procedure will. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. The sodium. Cold Pack Chemical Reaction Equation.
From www.pinterest.co.uk
Cold Pack Chemistry Exploring Endothermic and Exothermic Reactions Cold Pack Chemical Reaction Equation learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. Squeezing the cold pack breaks an inner bag of water, and the water. Cold Pack Chemical Reaction Equation.
From www.thoughtco.com
Make a Cold Pack from Hot Ice Cold Pack Chemical Reaction Equation learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in an endothermic reaction, a substance takes heat from its surroundings. So how does it work? The sodium thiosulfate needs energy to. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated. Cold Pack Chemical Reaction Equation.
From chemwiki.ucdavis.edu
13.1 Factors Affecting Solution Formation Chemwiki Cold Pack Chemical Reaction Equation A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. in an endothermic reaction, a substance takes heat from its surroundings. The sodium thiosulfate needs energy to. making an instant cold pack introduction ever used a cold pack on an injury?. Cold Pack Chemical Reaction Equation.
From sciencenotes.org
Hand Warmer Chemistry Easy Chemical Hot Packs Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. This quick and easy procedure will. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. So. Cold Pack Chemical Reaction Equation.
From www.numerade.com
SOLVEDGive the chemical equations for (a) the conversion of carbon Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. making an instant cold pack introduction. Cold Pack Chemical Reaction Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For The Dissociation Of Calcium Hydroxide In Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. This quick and easy procedure will. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. So how does it work? A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin. Cold Pack Chemical Reaction Equation.
From sms-jamaica.com
Instant Hot and Cold Packs Scientific & Medical Supplies Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. So how does it work? This quick and easy procedure will. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin. Cold Pack Chemical Reaction Equation.
From sciencenotes.org
Endothermic Reactions Definition and Examples Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. in an endothermic reaction, a substance takes heat from its surroundings. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and. Cold Pack Chemical Reaction Equation.
From sciencenotes.org
Hot and Cold Volcano Easy Endothermic and Exothermic Reactions Cold Pack Chemical Reaction Equation Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold. Cold Pack Chemical Reaction Equation.
From www.coursehero.com
[Solved] Virtual Lab Hot and Cold Packs Substance Equation for Cold Pack Chemical Reaction Equation In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. in an endothermic reaction, a substance takes heat from its surroundings. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. A cold pack consists of water (h. Cold Pack Chemical Reaction Equation.
From www.thinkswap.com
Endothermic Reaction Practical (Science of Cold Packs) Chemistry Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. So how does it work? in an endothermic reaction, a substance takes heat from its surroundings. making an instant cold pack introduction ever used a cold pack on an injury? A cold. Cold Pack Chemical Reaction Equation.
From slidetodoc.com
Solubility A solution is a homogeneous mixture that Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. So how does it work? A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. in. Cold Pack Chemical Reaction Equation.
From studylib.net
Chem32a_Hot Pack_30oct13 Cold Pack Chemical Reaction Equation making an instant cold pack introduction ever used a cold pack on an injury? learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. This quick and easy procedure will. in an endothermic reaction, a substance takes heat from its surroundings. Squeezing the cold pack breaks an inner. Cold Pack Chemical Reaction Equation.
From ubicaciondepersonas.cdmx.gob.mx
Chemical Cold Pack ubicaciondepersonas.cdmx.gob.mx Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. making an instant cold pack introduction ever used a cold pack on an injury? A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3). Cold Pack Chemical Reaction Equation.
From technology.techwallp.xyz
Types of Chemical Reactions With Examples Cold Pack Chemical Reaction Equation making an instant cold pack introduction ever used a cold pack on an injury? This quick and easy procedure will. In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. The sodium thiosulfate needs energy to. in an endothermic reaction, a substance takes heat from its surroundings. So how does. Cold Pack Chemical Reaction Equation.
From www.chemistryland.com
Lab 9 Double Replacement Reactions Cold Pack Chemical Reaction Equation Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. making an instant cold pack introduction ever used a cold pack on an injury? learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. A cold pack consists of. Cold Pack Chemical Reaction Equation.
From www.numerade.com
SOLVED Commercial cold packs and hot packs are available for treating Cold Pack Chemical Reaction Equation So how does it work? The sodium thiosulfate needs energy to. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. in. Cold Pack Chemical Reaction Equation.
From exomzvkxi.blob.core.windows.net
When Should You Use A Cold Pack at Gregory Hansen blog Cold Pack Chemical Reaction Equation learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. making an instant cold pack introduction ever used a cold pack on an injury? Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. The sodium thiosulfate needs energy. Cold Pack Chemical Reaction Equation.
From fphoto.photoshelter.com
science chemical reaction endothermic cold pack Fundamental Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. So how does it work? Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. This quick and easy procedure will. in an. Cold Pack Chemical Reaction Equation.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Cold Pack Chemical Reaction Equation Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. in an endothermic reaction, a substance takes heat from its surroundings. The sodium thiosulfate needs energy to. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction. Cold Pack Chemical Reaction Equation.
From courses.lumenlearning.com
The Dissolving Process CHEM 1305 Introductory Chemistry Cold Pack Chemical Reaction Equation in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. In this chemistry science fair project, you will investigate the. Cold Pack Chemical Reaction Equation.
From brainly.in
Sort the chemical reactions based on whether they absorb or release Cold Pack Chemical Reaction Equation In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. The sodium thiosulfate needs energy to. This quick and easy procedure will. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. in an. Cold Pack Chemical Reaction Equation.
From www.pinterest.com
Instant Ice Packs(Temperature) Inside an instant ice pack, there are Cold Pack Chemical Reaction Equation So how does it work? making an instant cold pack introduction ever used a cold pack on an injury? A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. Squeezing the cold pack breaks an inner bag of water, and the water. Cold Pack Chemical Reaction Equation.
From slidetodoc.com
Solubility A solution is a homogeneous mixture that Cold Pack Chemical Reaction Equation learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in an endothermic reaction, a substance takes heat from its surroundings. This quick and easy procedure will. The sodium thiosulfate needs energy to. making an instant cold pack introduction ever used a cold pack on an injury? A. Cold Pack Chemical Reaction Equation.
From www.alamy.com
Ammonium nitrate hires stock photography and images Alamy Cold Pack Chemical Reaction Equation in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. making an instant cold pack introduction ever used a cold pack on an injury? Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. A cold. Cold Pack Chemical Reaction Equation.
From gioqyutil.blob.core.windows.net
Corn Flour Chemical Formula at Jenny Dunning blog Cold Pack Chemical Reaction Equation The sodium thiosulfate needs energy to. In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. in an endothermic reaction, a substance takes heat from its surroundings. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. Squeezing. Cold Pack Chemical Reaction Equation.
From rainbowtech.net
79014 Chemical Cold Pack Rainbow Technology Cold Pack Chemical Reaction Equation learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in an endothermic reaction, a substance takes heat from its surroundings. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. A cold pack consists of water (h 2. Cold Pack Chemical Reaction Equation.
From ar.inspiredpencil.com
Endothermic And Exothermic Reaction Examples Cold Pack Chemical Reaction Equation in an endothermic reaction, a substance takes heat from its surroundings. In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. This quick and easy procedure will. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. Squeezing the cold pack. Cold Pack Chemical Reaction Equation.
From classfullkatmandu.z19.web.core.windows.net
Chemical Reactions And Equation Notes Cold Pack Chemical Reaction Equation in an endothermic reaction, a substance takes heat from its surroundings. Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. learn about the chemical reactions used to make cold. Cold Pack Chemical Reaction Equation.
From www.chegg.com
Solved Background. instant cold packs are activated when two Cold Pack Chemical Reaction Equation in an endothermic reaction, a substance takes heat from its surroundings. So how does it work? This quick and easy procedure will. A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. making an instant cold pack introduction ever used a. Cold Pack Chemical Reaction Equation.
From www.youtube.com
Instant Cold Pack The Chemicals Inside Product Breakdown YouTube Cold Pack Chemical Reaction Equation in an endothermic reaction, a substance takes heat from its surroundings. making an instant cold pack introduction ever used a cold pack on an injury? Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. So how does it work? The sodium thiosulfate needs energy to. learn. Cold Pack Chemical Reaction Equation.
From www.chemistrylearner.com
Heat (Enthalpy) of Solution Definition, Formula, & Problems Cold Pack Chemical Reaction Equation A cold pack consists of water (h 2 o) and ammonium nitrate (nh 4 no 3) separated by a thin film and placed in a plastic bag. making an instant cold pack introduction ever used a cold pack on an injury? So how does it work? in an endothermic reaction, a substance takes heat from its surroundings. Squeezing. Cold Pack Chemical Reaction Equation.
From www.slideserve.com
PPT conquer the Chemistry EOC Balancing Equations and Stoichiometry Cold Pack Chemical Reaction Equation making an instant cold pack introduction ever used a cold pack on an injury? In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs. in an endothermic reaction, a substance takes heat from its surroundings. learn about the chemical reactions used to make cold packs and which chemicals to. Cold Pack Chemical Reaction Equation.
From www.slideserve.com
PPT Cryotherapy & Thermotherapy PowerPoint Presentation, free Cold Pack Chemical Reaction Equation So how does it work? in an endothermic reaction, a substance takes heat from its surroundings. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. The sodium thiosulfate needs energy to. Squeezing the cold pack breaks an inner bag of water, and the water mixes. Cold Pack Chemical Reaction Equation.