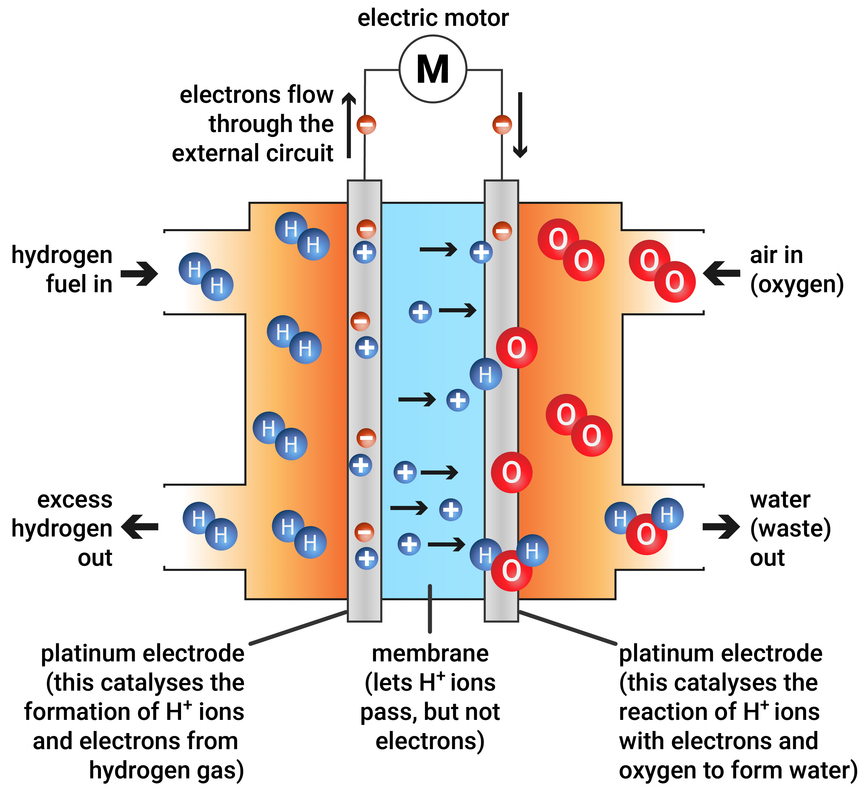
Fuel Cell Physics Definition . a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. In a fuel cell, hydrogen and oxygen are combined to generate. It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. what is a fuel cell?
from revisechemistry.uk
what is a fuel cell? a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. It is defined as an electrochemical cell that generates electrical energy from fuel via.
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk
Fuel Cell Physics Definition In a fuel cell, hydrogen and oxygen are combined to generate. It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In a fuel cell, hydrogen and oxygen are combined to generate. what is a fuel cell?
From www.pinterest.com
Schematic of a CNT composite hydrogen fuel cell. Hydrogen fuel cell Fuel Cell Physics Definition a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. In a fuel cell, hydrogen and oxygen are combined. Fuel Cell Physics Definition.
From www.slideserve.com
PPT Fuel Cells as Viable Electrical Sources PowerPoint Presentation Fuel Cell Physics Definition what is a fuel cell? It is defined as an electrochemical cell that generates electrical energy from fuel via. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell consists of two electrodes—a. Fuel Cell Physics Definition.
From manualdiagramausterlitz.z19.web.core.windows.net
Fuel Cell Schematic Diagram Fuel Cell Physics Definition a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. what is a fuel cell? fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy.. Fuel Cell Physics Definition.
From lylagokejames.blogspot.com
Describe How a Fuel Cell Works Fuel Cell Physics Definition what is a fuel cell? In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. a fuel cell consists. Fuel Cell Physics Definition.
From mungfali.com
A380 Fuel Cell Diagram Fuel Cell Physics Definition a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. what is a fuel cell? In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. It is defined as an electrochemical. Fuel Cell Physics Definition.
From www.alamy.com
Fuel cell diagram. Vector. Device that converts chemical potential Fuel Cell Physics Definition a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells (fcs) have been mentioned as an alternative energy technology. Fuel Cell Physics Definition.
From www.researchgate.net
Working principles of a) glycerol/O2 fuel cell and b) electrolysis cell Fuel Cell Physics Definition In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a galvanic cell or electrochemical power source,. Fuel Cell Physics Definition.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Fuel Cell Physics Definition what is a fuel cell? fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. It is defined as an. Fuel Cell Physics Definition.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cell Physics Definition a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and. Fuel Cell Physics Definition.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Fuel Cell Physics Definition a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells (fcs) have been mentioned. Fuel Cell Physics Definition.
From cafcp.org
Frequently Asked Questions California Fuel Cell Partnership Fuel Cell Physics Definition a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In a fuel cell, hydrogen and oxygen are combined to generate. It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel cell is a device that generates electricity through an electrochemical reaction,. Fuel Cell Physics Definition.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cell Physics Definition a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. fuel cells (fcs) have been mentioned. Fuel Cell Physics Definition.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Fuel Cell Physics Definition In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. what is a fuel cell? fuel cells (fcs) have been. Fuel Cell Physics Definition.
From www.nextbigfuture.com
Molten Carbonate High Temperature Fuel Cells Getting to Scale Fuel Cell Physics Definition a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. what is a fuel cell?. Fuel Cell Physics Definition.
From www.yourelectricalguide.com
Fuel Cell Working Principle your electrical guide Fuel Cell Physics Definition It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. what is a fuel cell? a fuel cell is a galvanic. Fuel Cell Physics Definition.
From specialgasinstruments.com
Hydrogen Fuel Cell Definition, Types, & Use Cases Special Gas Fuel Cell Physics Definition a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. what is a fuel cell? a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel. Fuel Cell Physics Definition.
From thechemistrynotes.com
Fuel Cell Definition, Types, and Advantages Fuel Cell Physics Definition In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. It is defined as an electrochemical cell that generates electrical energy from fuel via. fuel cells (fcs) have been mentioned as an alternative energy technology in which. Fuel Cell Physics Definition.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cell Physics Definition fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a galvanic cell or electrochemical power. Fuel Cell Physics Definition.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell Physics Definition fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. In. Fuel Cell Physics Definition.
From energyeducation.ca
Fuel cell Energy Education Fuel Cell Physics Definition a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. It. Fuel Cell Physics Definition.
From specialgasinstruments.com
Hydrogen Fuel Cell Definition, Types, & Use Cases Special Gas Fuel Cell Physics Definition It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells (fcs) have been mentioned as an. Fuel Cell Physics Definition.
From www.researchgate.net
Overview of the various types of fuel cells with the following Fuel Cell Physics Definition fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. . Fuel Cell Physics Definition.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS Fuel Cell Physics Definition what is a fuel cell? a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. It is defined as an electrochemical cell that generates electrical energy from fuel via. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell or electrochemical power source, which. Fuel Cell Physics Definition.
From www.myelectrical2015.com
Electrical Revolution Working Principle of Fuel Cell Fuel Cell Physics Definition what is a fuel cell? a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell consists of. Fuel Cell Physics Definition.
From www.youtube.com
Introduction to Types of Fuel Cells YouTube Fuel Cell Physics Definition fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. what is a fuel cell? a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode. Fuel Cell Physics Definition.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell Physics Definition a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. In a fuel cell, hydrogen and. Fuel Cell Physics Definition.
From gamesmartz.com
Fuel Cell Definition & Image GameSmartz Fuel Cell Physics Definition a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. In a fuel cell, hydrogen and. Fuel Cell Physics Definition.
From www.slideserve.com
PPT Fuel Cells for a Sustainable Energy Future PowerPoint Fuel Cell Physics Definition In a fuel cell, hydrogen and oxygen are combined to generate. what is a fuel cell? a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. . Fuel Cell Physics Definition.
From www.researchgate.net
A typical fuel cell stack design and components Download Scientific Fuel Cell Physics Definition a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells (fcs) have been mentioned. Fuel Cell Physics Definition.
From www.nfcrc.uci.edu
National Fuel Cell Research Center (NFCRC), UC Irvine Fuel Cell Physics Definition It is defined as an electrochemical cell that generates electrical energy from fuel via. what is a fuel cell? a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a galvanic cell. Fuel Cell Physics Definition.
From vajiramandravi.com
Fuel Cell Definition, Types, Advantages, Applications Fuel Cell Physics Definition It is defined as an electrochemical cell that generates electrical energy from fuel via. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. In a fuel cell, hydrogen and oxygen are combined to generate. what is a fuel cell? a fuel cell consists of two electrodes—a negative electrode (or anode). Fuel Cell Physics Definition.
From www.dreamstime.com
Hydrogen Fuel Cells. Education Infographic. Vector Design. Stock Vector Fuel Cell Physics Definition a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. what is a fuel cell? a fuel cell is a galvanic cell or electrochemical. Fuel Cell Physics Definition.
From www.comsol.de
4 Examples of Fuel Cell Modeling in COMSOL Multiphysics® COMSOL Blog Fuel Cell Physics Definition a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. It is defined as an electrochemical cell that generates electrical energy from fuel via. what is a fuel cell? In a fuel. Fuel Cell Physics Definition.
From www.e-conversion.de
Fuel cell principle econversion Fuel Cell Physics Definition It is defined as an electrochemical cell that generates electrical energy from fuel via. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. In a fuel cell, hydrogen and oxygen are combined to generate. what is a fuel cell? a fuel cell is a device that. Fuel Cell Physics Definition.
From www.slideserve.com
PPT Chemistry for Fuel Cells PowerPoint Presentation, free download Fuel Cell Physics Definition what is a fuel cell? fuel cells (fcs) have been mentioned as an alternative energy technology in which the chemical energy. a fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion.. Fuel Cell Physics Definition.