Test Method Validation Requirements . a description of the basic principles of the analytical test/technology (i.e., separation,. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. a validation study is designed to provide sufficient evidence that the analytical procedure meets. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument.
from fivevalidation.com
it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. a validation study is designed to provide sufficient evidence that the analytical procedure meets.
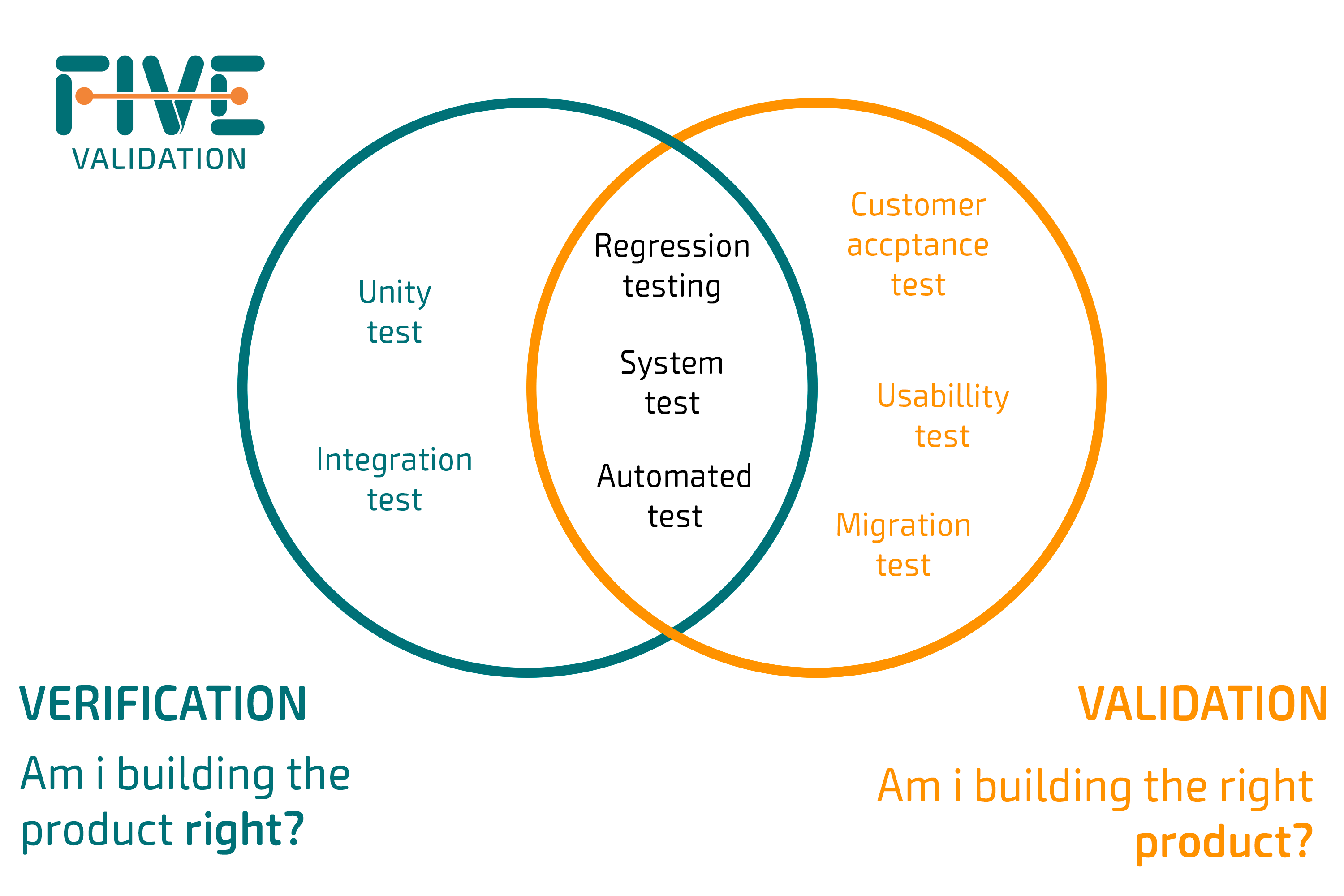
Verification & Validation V&V FIVE Validation
Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. a validation study is designed to provide sufficient evidence that the analytical procedure meets. a description of the basic principles of the analytical test/technology (i.e., separation,.
From www.template.net
Validation Report Templates 9+ Free Word, PDF Format Download Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. a validation study is designed to provide sufficient evidence that the analytical procedure meets. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. verification that. Test Method Validation Requirements.
From mungfali.com
Test Method Validation Test Method Validation Requirements a validation study is designed to provide sufficient evidence that the analytical procedure meets. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. laboratories are. Test Method Validation Requirements.
From www.sampletemplates.com
FREE 9+ Sample Validation Plan Templates in PDF MS Word Test Method Validation Requirements a validation study is designed to provide sufficient evidence that the analytical procedure meets. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. section 3 “validation tests, methodology and evaluation” summarises. Test Method Validation Requirements.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Test Method Validation Requirements verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. a validation study is designed to provide sufficient evidence that the analytical procedure meets. it provides guidance and recommendations on how to. Test Method Validation Requirements.
From www.researchgate.net
Summary of requirements validation techniques. Download Table Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. a description of the basic principles of the analytical test/technology (i.e., separation,. . Test Method Validation Requirements.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Test Method Validation Requirements a validation study is designed to provide sufficient evidence that the analytical procedure meets. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. it provides guidance and recommendations on how to derive and evaluate the various validation tests. Test Method Validation Requirements.
From dxofqtloz.blob.core.windows.net
Test Method Validation Vs Gage R&R at Jeffrey Rutherford blog Test Method Validation Requirements it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. a validation study is designed to provide sufficient evidence that the analytical procedure meets. section 3 “validation tests, methodology and evaluation” summarises. Test Method Validation Requirements.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. it provides guidance and recommendations on how to derive and evaluate the various validation tests. Test Method Validation Requirements.
From lgmpharma.com
Best Practices For Successful Method Validation LGM Pharma Test Method Validation Requirements verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. a description of the basic principles of the analytical test/technology (i.e., separation,. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. section 3 “validation tests, methodology and evaluation” summarises the typical. Test Method Validation Requirements.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. a description of the basic principles. Test Method Validation Requirements.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Test Method Validation Requirements verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. it provides guidance and recommendations on how to derive and evaluate the various validation tests. Test Method Validation Requirements.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. section 3 “validation tests, methodology and. Test Method Validation Requirements.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Test Method Validation Requirements a validation study is designed to provide sufficient evidence that the analytical procedure meets. a description of the basic principles of the analytical test/technology (i.e., separation,. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. . Test Method Validation Requirements.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a validation study is designed to provide sufficient evidence that the analytical procedure meets. a description of the basic principles of the analytical test/technology (i.e., separation,. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that. Test Method Validation Requirements.
From www.collidu.com
Requirement Validation PowerPoint Presentation Slides PPT Template Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence. Test Method Validation Requirements.
From present5.com
Validation and verification Requirements of ISO IEC 17025 by Test Method Validation Requirements verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. a validation study is designed to provide sufficient evidence that the analytical procedure meets. it provides guidance and recommendations on how to. Test Method Validation Requirements.
From www.lambdatest.com
What is Verification and Validation in Software Testing? LambdaTest Test Method Validation Requirements a description of the basic principles of the analytical test/technology (i.e., separation,. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. it provides guidance and recommendations on how to derive and. Test Method Validation Requirements.
From ppt-online.org
Method Validation and Verification Protocols for Test Methods Test Method Validation Requirements a description of the basic principles of the analytical test/technology (i.e., separation,. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or. Test Method Validation Requirements.
From www.pmv-groupe.com
Testing as a requirements validation method PMV Groupe Mag' PMV Groupe Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a validation study is designed to provide sufficient evidence that the analytical procedure meets. verification that a laboratory can adequately operate a standard method requires that the laboratory provide. Test Method Validation Requirements.
From fivevalidation.com
Verification & Validation V&V FIVE Validation Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. a validation study is designed to provide sufficient evidence that the analytical procedure meets. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that. Test Method Validation Requirements.
From www.slideserve.com
PPT Requirements Validation PowerPoint Presentation, free download Test Method Validation Requirements verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. a description of the basic principles of the analytical test/technology (i.e., separation,. a validation study is designed to provide sufficient evidence that. Test Method Validation Requirements.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. a description of the basic principles of the analytical test/technology (i.e., separation,. verification that a laboratory can adequately operate a standard method requires that. Test Method Validation Requirements.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Test Method Validation Requirements a description of the basic principles of the analytical test/technology (i.e., separation,. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. it provides guidance and recommendations on how to derive and. Test Method Validation Requirements.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a validation study is designed to provide sufficient evidence that the analytical procedure meets. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that a laboratory can adequately operate a standard method requires that the laboratory provide. Test Method Validation Requirements.
From techblogs.42gears.com
Verification and Validation in Software Testing Tech Blogs Test Method Validation Requirements a description of the basic principles of the analytical test/technology (i.e., separation,. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. . Test Method Validation Requirements.
From www.slideserve.com
PPT Requirements Verification and Validation PowerPoint Presentation Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a validation study is designed to provide sufficient evidence that the analytical procedure meets. a description of the basic principles of the analytical test/technology (i.e., separation,. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. verification that. Test Method Validation Requirements.
From www.testgorilla.com
A brief introduction to Test validation Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. a validation study is designed to provide sufficient evidence that the analytical procedure meets. a description of the basic principles of the analytical test/technology (i.e., separation,. it provides. Test Method Validation Requirements.
From www.slideserve.com
PPT Requirements Validation PowerPoint Presentation, free download Test Method Validation Requirements a description of the basic principles of the analytical test/technology (i.e., separation,. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. a validation study is designed to provide sufficient evidence that the analytical procedure meets. . Test Method Validation Requirements.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. a description of the basic principles of the analytical test/technology (i.e., separation,. section 3 “validation tests, methodology and evaluation” summarises the typical. Test Method Validation Requirements.
From www.softwaretestinghelp.com
Validation Testing Ultimate Guide Test Method Validation Requirements section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. a description of the basic principles of the analytical test/technology (i.e., separation,. a validation study is designed to provide sufficient evidence that the analytical procedure meets. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. it provides. Test Method Validation Requirements.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. a validation study is designed to provide sufficient evidence that the analytical procedure meets. a description of the basic principles of the analytical test/technology (i.e., separation,. it provides guidance and recommendations on how to derive and evaluate the various validation. Test Method Validation Requirements.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Test Method Validation Requirements verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. a validation study is designed to provide sufficient evidence that the analytical procedure meets. a description of the basic principles of the analytical test/technology (i.e., separation,. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. . Test Method Validation Requirements.
From dxofqtloz.blob.core.windows.net
Test Method Validation Vs Gage R&R at Jeffrey Rutherford blog Test Method Validation Requirements a validation study is designed to provide sufficient evidence that the analytical procedure meets. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. a description of the basic principles of the analytical test/technology (i.e., separation,. verification that a laboratory can adequately operate a standard method requires that the laboratory. Test Method Validation Requirements.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Requirements a validation study is designed to provide sufficient evidence that the analytical procedure meets. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. verification that a laboratory can adequately operate a standard method requires that the laboratory provide. Test Method Validation Requirements.
From www.technolush.com
Verification & Validation Model TechnoLush Test Method Validation Requirements laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. section 3 “validation tests, methodology and evaluation” summarises the typical methodologies. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. a description of the basic principles of the analytical test/technology (i.e., separation,. . Test Method Validation Requirements.