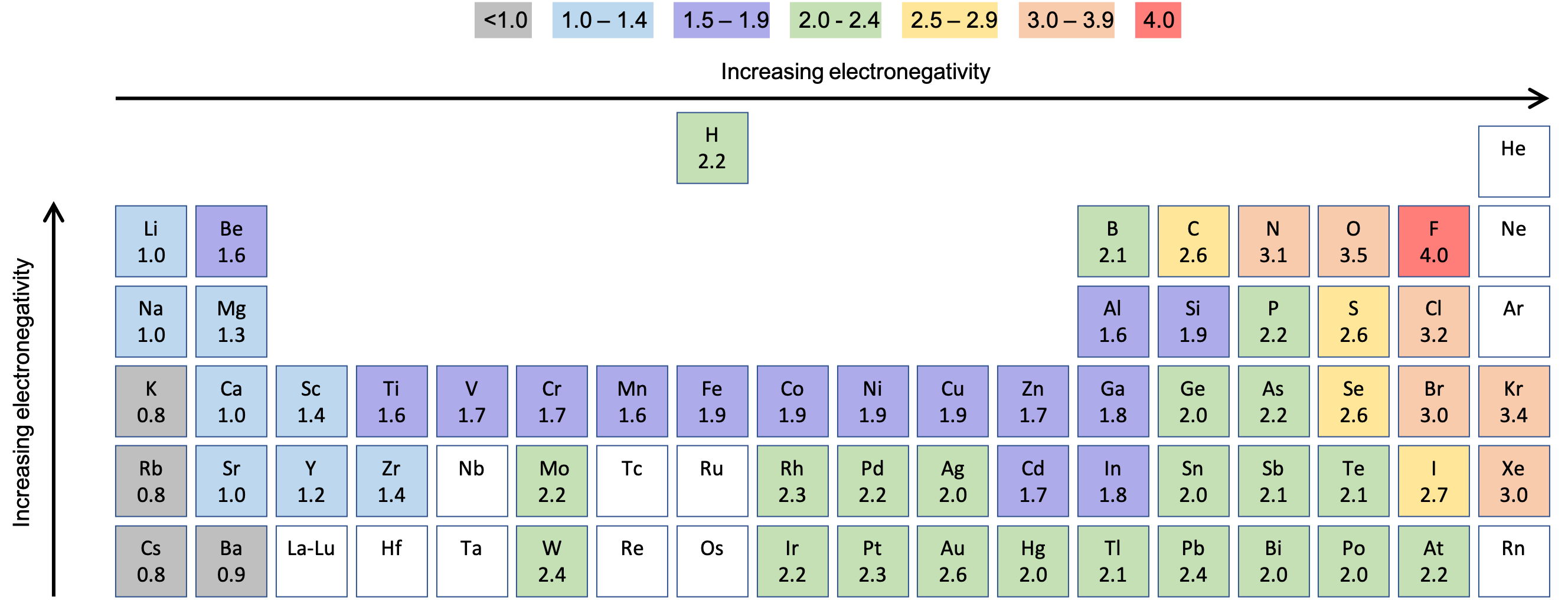
Chloride Low Electronegativity . It can also be used to predict if the. in chemistry, electronegativity is a measure of how strongly an atom. select the electronegativity value of the first element. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Select the electronegativity value of the second element. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: The pauling scale is the. It is caused by the attractive.
from wisc.pb.unizin.org
this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. The pauling scale is the. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. in chemistry, electronegativity is a measure of how strongly an atom. It is caused by the attractive. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. select the electronegativity value of the first element. It can also be used to predict if the. Select the electronegativity value of the second element.
Bonding and Electronegativity (M8Q1) UWMadison Chemistry 103/104
Chloride Low Electronegativity electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. It can also be used to predict if the. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: in chemistry, electronegativity is a measure of how strongly an atom. Select the electronegativity value of the second element. select the electronegativity value of the first element. It is caused by the attractive. The pauling scale is the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts Chloride Low Electronegativity electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the. in chemistry, electronegativity is a measure of how strongly an atom. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. 119 rows. Chloride Low Electronegativity.
From www.shutterstock.com
Electronegativity Infographic Diagram Example Sodium Chloride Stock Chloride Low Electronegativity electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the. Select the electronegativity value of the second element. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. in chemistry, electronegativity is a measure. Chloride Low Electronegativity.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Chloride Low Electronegativity electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: select the electronegativity value of the first element. It can also be used to predict if. Chloride Low Electronegativity.
From www.wikihow.com
4 Ways to Calculate Electronegativity wikiHow Chloride Low Electronegativity select the electronegativity value of the first element. It is caused by the attractive. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent.. Chloride Low Electronegativity.
From wisc.pb.unizin.org
Bonding and Electronegativity (M8Q1) UWMadison Chemistry 103/104 Chloride Low Electronegativity It is caused by the attractive. in chemistry, electronegativity is a measure of how strongly an atom. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electronegativity is a. Chloride Low Electronegativity.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Chloride Low Electronegativity electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. in chemistry, electronegativity is a measure of how strongly an atom. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between. Chloride Low Electronegativity.
From www.nanowerk.com
Electronegativity explained Chloride Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. in chemistry, electronegativity is a measure of how strongly an atom. The pauling scale is the. select the electronegativity value of the first element. electronegativity is the tendency of an atom to attract a pair of electrons in a. Chloride Low Electronegativity.
From student-tutor.com
Making Sense of the Electronegativity Chart StudentTutor Education Blog Chloride Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is caused by the attractive. Select the electronegativity value of the second element. electronegativity is the tendency of an atom to attract a pair of electrons in. Chloride Low Electronegativity.
From www.periodictableprintable.com
Periodic Table Of Elements Electronegativity 2024 Periodic Table Chloride Low Electronegativity The pauling scale is the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. in chemistry, electronegativity is a measure of how strongly an atom.. Chloride Low Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chloride Low Electronegativity select the electronegativity value of the first element. It is caused by the attractive. The pauling scale is the. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Select the electronegativity value of the second element. in chemistry, electronegativity is a measure of how strongly an atom. It can. Chloride Low Electronegativity.
From chemistry.com.pk
electronegativity chart Chloride Low Electronegativity in chemistry, electronegativity is a measure of how strongly an atom. It is caused by the attractive. select the electronegativity value of the first element. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity is the tendency of an atom to attract a pair of electrons in. Chloride Low Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Chloride Low Electronegativity Select the electronegativity value of the second element. It can also be used to predict if the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. select the electronegativity value of the first element. It is caused. Chloride Low Electronegativity.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Chloride Low Electronegativity select the electronegativity value of the first element. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is caused by the attractive. It can also be used to predict if the. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. to predict the polarity. Chloride Low Electronegativity.
From sciencenotes.org
Electronegativity Definition and Trend Chloride Low Electronegativity in chemistry, electronegativity is a measure of how strongly an atom. The pauling scale is the. Select the electronegativity value of the second element. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. It can also be. Chloride Low Electronegativity.
From ar.inspiredpencil.com
Periodic Table With Electronegativity And Electron Configuration Chloride Low Electronegativity in chemistry, electronegativity is a measure of how strongly an atom. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. It can also be used to predict if the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: It. Chloride Low Electronegativity.
From sciencenotes.org
Electronegativity Chart PDF Chloride Low Electronegativity in chemistry, electronegativity is a measure of how strongly an atom. The pauling scale is the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: It is caused by the attractive. 119 rows electronegativity is used to predict whether a bond between. Chloride Low Electronegativity.
From byjus.com
Electronegativity of An Element Least electronegative element Chloride Low Electronegativity in chemistry, electronegativity is a measure of how strongly an atom. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. select the electronegativity value of the first element. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is caused. Chloride Low Electronegativity.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID6836286 Chloride Low Electronegativity electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. It can also be used to predict if the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. in. Chloride Low Electronegativity.
From mavink.com
Printable Electronegativity Table Chloride Low Electronegativity It is caused by the attractive. select the electronegativity value of the first element. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. to predict the polarity of the bonds in. Chloride Low Electronegativity.
From revisionscience.com
Electronegativity ALevel Chemistry Chloride Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. It can also be used to predict if the. electronegativity is a measure of the tendency of an atom to attract a bonding. Chloride Low Electronegativity.
From mungfali.com
19+ Electronegativity Chart Templates Free Sample, Example, Format D0B Chloride Low Electronegativity It is caused by the attractive. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. select the electronegativity value of the first element. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: this. Chloride Low Electronegativity.
From chirp1.chem.ubc.ca
4.1 Electronegativity ChIRP Chloride Low Electronegativity electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. The pauling scale is the. select the electronegativity value of the first element. to predict the polarity of the bonds in cl 2, hcl, and nacl, for. Chloride Low Electronegativity.
From therealgroupichem.weebly.com
Electronegativity Group i Chemistry(iiiescuro) Chloride Low Electronegativity electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: It is caused by the attractive. It can also be used to predict if the. The pauling. Chloride Low Electronegativity.
From www.numerade.com
SOLVED For the compounds HCI and SO2, with the element information Chloride Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. in chemistry, electronegativity is a measure of how strongly an atom. It can also be used to predict if the. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. The pauling scale is the. Select the electronegativity. Chloride Low Electronegativity.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Chloride Low Electronegativity The pauling scale is the. Select the electronegativity value of the second element. It can also be used to predict if the. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. 119 rows electronegativity is used to. Chloride Low Electronegativity.
From thechemistrynotes.com
Difference Between Electronegativity and Electron affinity Chloride Low Electronegativity select the electronegativity value of the first element. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. Select the electronegativity value of the second element. It can also be used to predict if the. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. electronegativity is. Chloride Low Electronegativity.
From mungfali.com
Electronegativity Chart And Lewis Structures Chloride Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. It can also be used to predict if the. select the electronegativity value of the first element. in chemistry, electronegativity is a measure of how strongly an atom. Select the electronegativity value of the second element. to predict the polarity of the bonds in. Chloride Low Electronegativity.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Chloride Low Electronegativity The pauling scale is the. Select the electronegativity value of the second element. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: electronegativity is the. Chloride Low Electronegativity.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Chloride Low Electronegativity select the electronegativity value of the first element. It is caused by the attractive. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: The pauling scale is the. in chemistry, electronegativity is a measure of how strongly an atom. 119 rows. Chloride Low Electronegativity.
From pubchem.ncbi.nlm.nih.gov
Electronegativity Periodic Table of Elements PubChem Chloride Low Electronegativity select the electronegativity value of the first element. It can also be used to predict if the. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. It is caused by the attractive. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Select the electronegativity value of. Chloride Low Electronegativity.
From www.science-revision.co.uk
Electronegativity and polar bonds Chloride Low Electronegativity select the electronegativity value of the first element. It can also be used to predict if the. It is caused by the attractive. The pauling scale is the. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. Select the electronegativity value of the second element. 119 rows electronegativity is used to predict whether a. Chloride Low Electronegativity.
From www.dreamstime.com
Electronegativity Periodic Table Stock Illustration Illustration of Chloride Low Electronegativity electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. It is caused by the attractive. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. Select the electronegativity value of. Chloride Low Electronegativity.
From issr.edu.kh
Electronegativity of polarity Chloride Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is caused by the attractive. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the. in chemistry, electronegativity is a measure of how. Chloride Low Electronegativity.
From general.chemistrysteps.com
CH3Cl Polar or Nonpolar Chemistry Steps Chloride Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is caused by the attractive. in chemistry, electronegativity is a measure of how strongly an atom. 119 rows electronegativity is used to predict whether a bond. Chloride Low Electronegativity.
From chemdictionary.org
Electronegativity Definition And Examples Chemistry Dictionary Chloride Low Electronegativity The pauling scale is the. in chemistry, electronegativity is a measure of how strongly an atom. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Select the electronegativity value of the second element. select the electronegativity. Chloride Low Electronegativity.