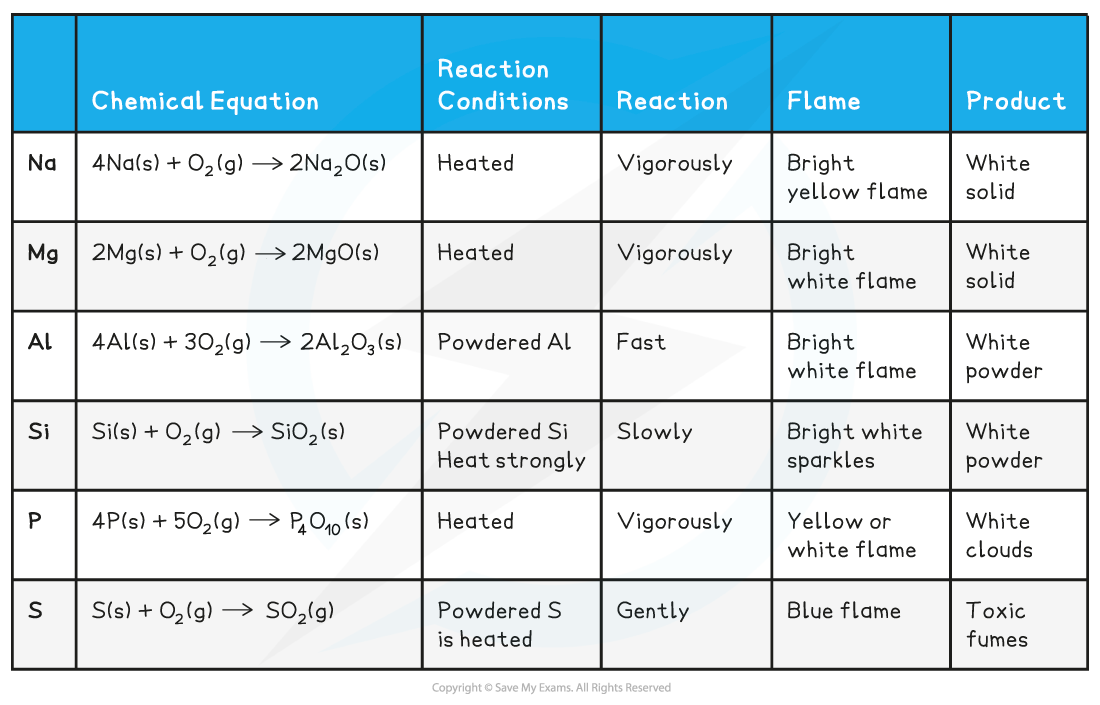
Chlorine Peroxide Formula . the peroxide equation is: The physical properties of the oxides. the oxidizing bleaches (and bleaching agents) in common use today are: Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na 2 o 2. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7.
from www.linstitute.net
the peroxide equation is: 2na + o 2 na 2 o 2. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. The physical properties of the oxides. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the oxidizing bleaches (and bleaching agents) in common use today are:
AQA A Level Chemistry复习笔记6.1.2 Reaction with Oxygen翰林国际教育
Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the oxidizing bleaches (and bleaching agents) in common use today are: We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na 2 o 2. The physical properties of the oxides. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the peroxide equation is:
From treatybottle13.pythonanywhere.com
Divine Equation For Hydrogen Peroxide Balanced Equations Photosynthesis Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the peroxide equation is: the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine. Chlorine Peroxide Formula.
From healingoracle.ch
THE HISTORY OF CHLORINE DIOXIDE Healing Oracle Chlorine Peroxide Formula Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the oxidizing bleaches (and bleaching agents) in common use today are: the peroxide equation is: We report ab initio calculations of the molecular structures of the various cl. Chlorine Peroxide Formula.
From segudanggambar875.blogspot.com
Hydrogen Peroxide And Manganese Dioxide Balanced Equation Hydrogen Chlorine Peroxide Formula the peroxide equation is: 2na + o 2 na 2 o 2. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. The physical properties of the oxides. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to. Chlorine Peroxide Formula.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: The physical properties of the oxides. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. We. Chlorine Peroxide Formula.
From www.tessshebaylo.com
Balanced Chemical Equation Of Hydrogen Peroxide And Ferric Chloride Chlorine Peroxide Formula Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na 2 o 2. the oxidizing bleaches (and bleaching agents) in. Chlorine Peroxide Formula.
From imgbin.com
Perchloryl Fluoride Chlorine Peroxide PNG, Clipart, Bromine, Caesium Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. The physical properties of the oxides. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation. Chlorine Peroxide Formula.
From www.ingridscience.ca
Hydrogen peroxide chemistry ingridscience.ca Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: the peroxide equation is: the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen. Chlorine Peroxide Formula.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Bleach Tessshebaylo Chlorine Peroxide Formula The physical properties of the oxides. the peroxide equation is: the oxidizing bleaches (and bleaching agents) in common use today are: Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. 2na + o 2 na 2 o. Chlorine Peroxide Formula.
From stock.adobe.com
Hydrogen Peroxide, perhydrol, H2O2 molecule. It is peroxide, oxidizing Chlorine Peroxide Formula Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. 2na + o 2 na 2 o 2. The physical properties of the oxides. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the. Chlorine Peroxide Formula.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. the oxidizing bleaches (and bleaching agents) in common use today are: 2na + o 2 na 2 o 2. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and. Chlorine Peroxide Formula.
From www.youtube.com
Bleach and Hydrogen Peroxide Reaction + Balanced Equation YouTube Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. 2na + o 2 na 2 o 2. chlorine forms. Chlorine Peroxide Formula.
From depositphotos.com
HNO2 nitrous acid molecule — Stock Vector © MariaShmitt 106911764 Chlorine Peroxide Formula We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. The physical properties of the oxides. 2na + o 2 na 2 o 2. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the peroxide equation. Chlorine Peroxide Formula.
From rescuedisinfectants.com
Accelerated Hydrogen Peroxide® (AHP®) versus Chlorine Dioxide Rescue Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na 2 o 2. The physical properties of the oxides. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the peroxide equation is: chlorine forms a. Chlorine Peroxide Formula.
From www.shutterstock.com
Cl2o2 Chlorine Peroxide Molecule Stock Vector Illustration 384669439 Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. the oxidizing bleaches (and bleaching agents) in common use today are: 2na + o 2 na 2 o 2. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. We report ab initio calculations of the molecular structures of the various cl 2 o. Chlorine Peroxide Formula.
From www.linstitute.net
AQA A Level Chemistry复习笔记6.1.2 Reaction with Oxygen翰林国际教育 Chlorine Peroxide Formula Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the peroxide equation is: the oxidizing bleaches (and bleaching agents) in common use today are: the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine. Chlorine Peroxide Formula.
From cleanhospital.com
Chlorine Dioxide Chlorine Peroxide Formula We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. The physical properties of the oxides. 2na + o 2 na 2 o 2. the peroxide equation is: chlorine forms a. Chlorine Peroxide Formula.
From www.docdroid.net
Chlorine Dioxide.pdf DocDroid Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na 2 o 2. the peroxide equation is: Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. chlorine forms. Chlorine Peroxide Formula.
From sjofictgvrlvelw.blogspot.com
What Is The Formula For Chlorine Dioxide Chlorine Peroxide Formula We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na 2 o 2. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the. Chlorine Peroxide Formula.
From www.essentialchemicalindustry.org
Chlorine Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the peroxide equation is: We report ab initio calculations of the molecular structures of the various cl. Chlorine Peroxide Formula.
From www.dreamstime.com
Chlorine Dioxide ClO2 Molecule. Used in Pulp Bleaching and for Chlorine Peroxide Formula 2na + o 2 na 2 o 2. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the peroxide equation is: The physical properties of the oxides. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition. Chlorine Peroxide Formula.
From www.sureaqua.com
Chlorine Dioxide in Water Treatment SureAqua Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na 2 o 2. the oxidizing bleaches (and bleaching agents) in common use today are: We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. The physical properties. Chlorine Peroxide Formula.
From www.dreamstime.com
Chlorine Dioxide, ClO2, Ballandstick Model, Molecular and Chemical Chlorine Peroxide Formula Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the peroxide equation is: the oxidizing bleaches (and bleaching. Chlorine Peroxide Formula.
From www.ingridscience.ca
Hydrogen peroxide chemistry ingridscience.ca Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. the peroxide equation is: 2na + o 2 na 2 o 2. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. The physical properties of the oxides.. Chlorine Peroxide Formula.
From www.youtube.com
Redox Reaction Hydrogen Peroxide (H2O2) With Chlorine Dioxide (ClO2 Chlorine Peroxide Formula the peroxide equation is: 2na + o 2 na 2 o 2. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the oxidizing bleaches (and bleaching agents) in common use today are: The physical properties of the oxides. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and. Chlorine Peroxide Formula.
From www.tessshebaylo.com
Hydrogen Peroxide And Iron 3 Chloride Balanced Equation Tessshebaylo Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the peroxide equation is: We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the oxidizing bleaches (and bleaching agents) in common use today. Chlorine Peroxide Formula.
From stock.adobe.com
Hydrogen Peroxide Structural Chemical Formula Model Stock Vector Chlorine Peroxide Formula We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the oxidizing bleaches (and bleaching agents) in common use today are: The physical properties of the oxides. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. 2na + o 2 na. Chlorine Peroxide Formula.
From www.dreamstime.com
Hydrogen Peroxide Molecule. Skeletal Formula. Stock Vector Chlorine Peroxide Formula the oxidizing bleaches (and bleaching agents) in common use today are: chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. The physical properties of the oxides. the peroxide equation is: Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. 2na + o 2 na 2 o. Chlorine Peroxide Formula.
From www.animalia-life.club
Bleach Chemical Formula Chlorine Peroxide Formula We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7.. Chlorine Peroxide Formula.
From www.youtube.com
How to Write the Formula for Chlorine dioxide YouTube Chlorine Peroxide Formula chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. The physical properties of the oxides. the oxidizing bleaches (and bleaching agents) in common use today are: We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,.. Chlorine Peroxide Formula.
From www.tessshebaylo.com
Balanced Chemical Equation Of Hydrogen Peroxide And Ferric Chloride Chlorine Peroxide Formula Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. 2na + o 2 na 2 o 2. We report ab initio. Chlorine Peroxide Formula.
From www.safesol.co.uk
Biofilm Chlorine Dioxide Versus HuwaSan (silver stabilised hydrogen Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. the peroxide equation is: Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. The physical properties of the oxides. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. 2na. Chlorine Peroxide Formula.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Chlorine Peroxide Formula the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. The physical properties of the oxides. We report ab initio calculations of the molecular structures of the various cl 2 o 2 isomers, transition states,. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation. Chlorine Peroxide Formula.
From www.shutterstock.com
Formula And Structure Of Chlorine Dioxide Molecule (Clo2)Main Chlorine Peroxide Formula chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the oxidizing bleaches (and bleaching agents) in common use today are:. Chlorine Peroxide Formula.
From draweasy8.blogspot.com
Hclo4 Lewis Structure Resonance Draw Easy Chlorine Peroxide Formula 2na + o 2 na 2 o 2. the oxidizing bleaches (and bleaching agents) in common use today are: The physical properties of the oxides. chlorine forms a series of oxides (table 10.3.2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. We report ab initio calculations of the molecular structures of the. Chlorine Peroxide Formula.
From www.shutterstock.com
Chlorine Peroxide Known Dichlorine Dioxide Clo ilustración de stock Chlorine Peroxide Formula Chlorine, chlorine dioxide, alkaline hypochlorites, hydrogen peroxide,. the chloryl chloride, chlorine peroxide clclo2 is observed to be stabilised with respect to the chlorine peroxide. the oxidizing bleaches (and bleaching agents) in common use today are: the peroxide equation is: The physical properties of the oxides. We report ab initio calculations of the molecular structures of the various. Chlorine Peroxide Formula.