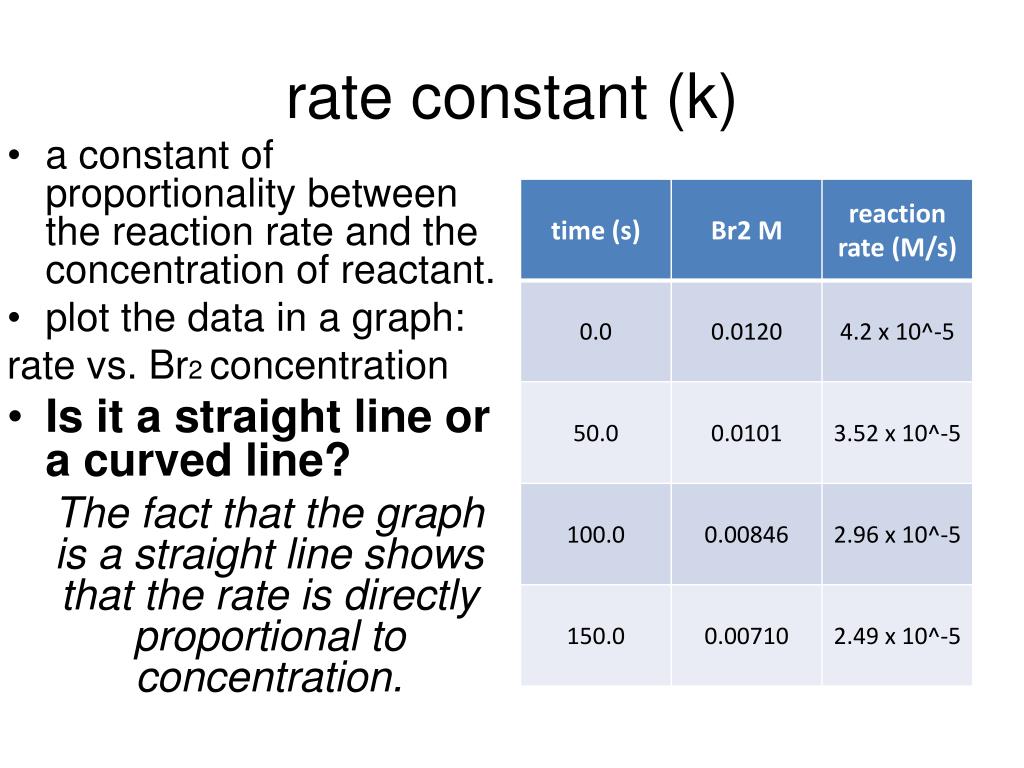
Standard Rate Constant . It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations.
from www.slideserve.com
It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction.
PPT Chemical PowerPoint Presentation, free download ID6305526
Standard Rate Constant the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction.
From www.doubtnut.com
What is the unit of rate constant of the reaction for which the above Standard Rate Constant the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the rate constant is a proportionality factor in the rate law of chemical kinetics that. Standard Rate Constant.
From slideplayer.com
Reaction Rates and Equilibrium ppt download Standard Rate Constant It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants. Standard Rate Constant.
From mavink.com
Units For Rate Constant Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the. Standard Rate Constant.
From www.youtube.com
rates, order and the rate constant YouTube Standard Rate Constant the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by. Standard Rate Constant.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Standard Rate Constant the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the standard electrochemical rate constant (k o) is the important. Standard Rate Constant.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID5609537 Standard Rate Constant if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter. Standard Rate Constant.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Standard Rate Constant the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. if the rate constant for a reaction is measure at two temperatures,. Standard Rate Constant.
From www.researchgate.net
Rate constant at different temperatures. Download Table Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by. Standard Rate Constant.
From gamesmartz.com
Specific Rate Constant Definition & Image GameSmartz Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction. Standard Rate Constant.
From www.doubtnut.com
The following rate data were obtained at 303 K for the following react Standard Rate Constant the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. if the rate constant for a reaction is measure at two temperatures, the. Standard Rate Constant.
From www.thetechedvocate.org
How to calculate rate constant The Tech Edvocate Standard Rate Constant It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the. Standard Rate Constant.
From www.researchgate.net
Rate constant vs temperature relation to determine the activation Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the. Standard Rate Constant.
From www.sarthaks.com
The above plot is for _______ order rection to calculate value of rate Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. . Standard Rate Constant.
From www.slideserve.com
PPT Chapter 14 Chemical PowerPoint Presentation, free Standard Rate Constant if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. It is also known as the reaction rate constant or reaction rate coefficient and is indicated. Standard Rate Constant.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID6305526 Standard Rate Constant It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of. Standard Rate Constant.
From www.slideserve.com
PPT SCH4U Unit 1 Energy Changes & Rates of Reactions (Cont’d Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants. Standard Rate Constant.
From www.pinterest.com
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Standard Rate Constant It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the standard electrochemical rate constant (k o) is the important parameter which quantifies. Standard Rate Constant.
From tasubtitlex.weebly.com
Ideal gas law weather calculator tasubtitleX Standard Rate Constant the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate constant or reaction rate coefficient and. Standard Rate Constant.
From www.teachertube.com
Constant Rate of Change Standard Rate Constant if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the standard electrochemical rate constant (k o) is the important parameter which quantifies. Standard Rate Constant.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper Standard Rate Constant the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. It is also known as the reaction rate constant or reaction rate coefficient and is. Standard Rate Constant.
From brainly.com
Which equation would be used to calculate the rate constant from Standard Rate Constant the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant is a proportionality factor in the rate law of chemical kinetics. Standard Rate Constant.
From www.pdfprof.com
rate constant equation Standard Rate Constant if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the rate constant is a proportionality factor in the rate law of chemical. Standard Rate Constant.
From www.chegg.com
Solved Calculate the rate constant with proper units. Standard Rate Constant the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k and the exponents m, n, and p must be. Standard Rate Constant.
From www.researchgate.net
Reaction rate constant at different temperature. Download Scientific Standard Rate Constant It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant is a proportionality factor in the rate law of chemical kinetics that. Standard Rate Constant.
From zuoti.pro
The rate constant for a certain reaction is k = 2.50×10−3 s−1 . If the Standard Rate Constant the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the rate constant is a proportionality factor in the rate law of chemical kinetics that. Standard Rate Constant.
From www.youtube.com
Units of rate constant YouTube Standard Rate Constant the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the standard electrochemical rate constant (k o) is the important parameter which quantifies. Standard Rate Constant.
From study.com
Rate Constant & Rate Law Definition, Differences & Examples Lesson Standard Rate Constant the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant is a proportionality factor in the rate law of chemical kinetics. Standard Rate Constant.
From www.slideserve.com
PPT Ch. 12 Rates and Rate Laws PowerPoint Presentation, free download Standard Rate Constant the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the standard electrochemical rate constant (k o) is the important parameter which quantifies the.. Standard Rate Constant.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID4206194 Standard Rate Constant It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. if the rate constant for a reaction is measure at two temperatures,. Standard Rate Constant.
From www.researchgate.net
Graph showing the dependence of rate constant on [KOEt] for the Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by. Standard Rate Constant.
From www.slideserve.com
PPT Rates and Rate Laws PowerPoint Presentation, free download ID Standard Rate Constant the rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the standard electrochemical rate constant (k o) is the important parameter which quantifies. Standard Rate Constant.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID496061 Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. . Standard Rate Constant.
From pediaa.com
Difference Between Rate of Reaction and Rate Constant Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. if the rate constant for a reaction is measure at two temperatures, the activation energy can be determined by taking the ratio. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of. Standard Rate Constant.
From www.researchgate.net
Dependencies of the rate constant k obs (1) and the Langmuir constant K Standard Rate Constant the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction. the standard electrochemical rate constant (k o) is the important parameter which quantifies the.. Standard Rate Constant.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Standard Rate Constant the standard electrochemical rate constant (k o) is the important parameter which quantifies the. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction.. Standard Rate Constant.