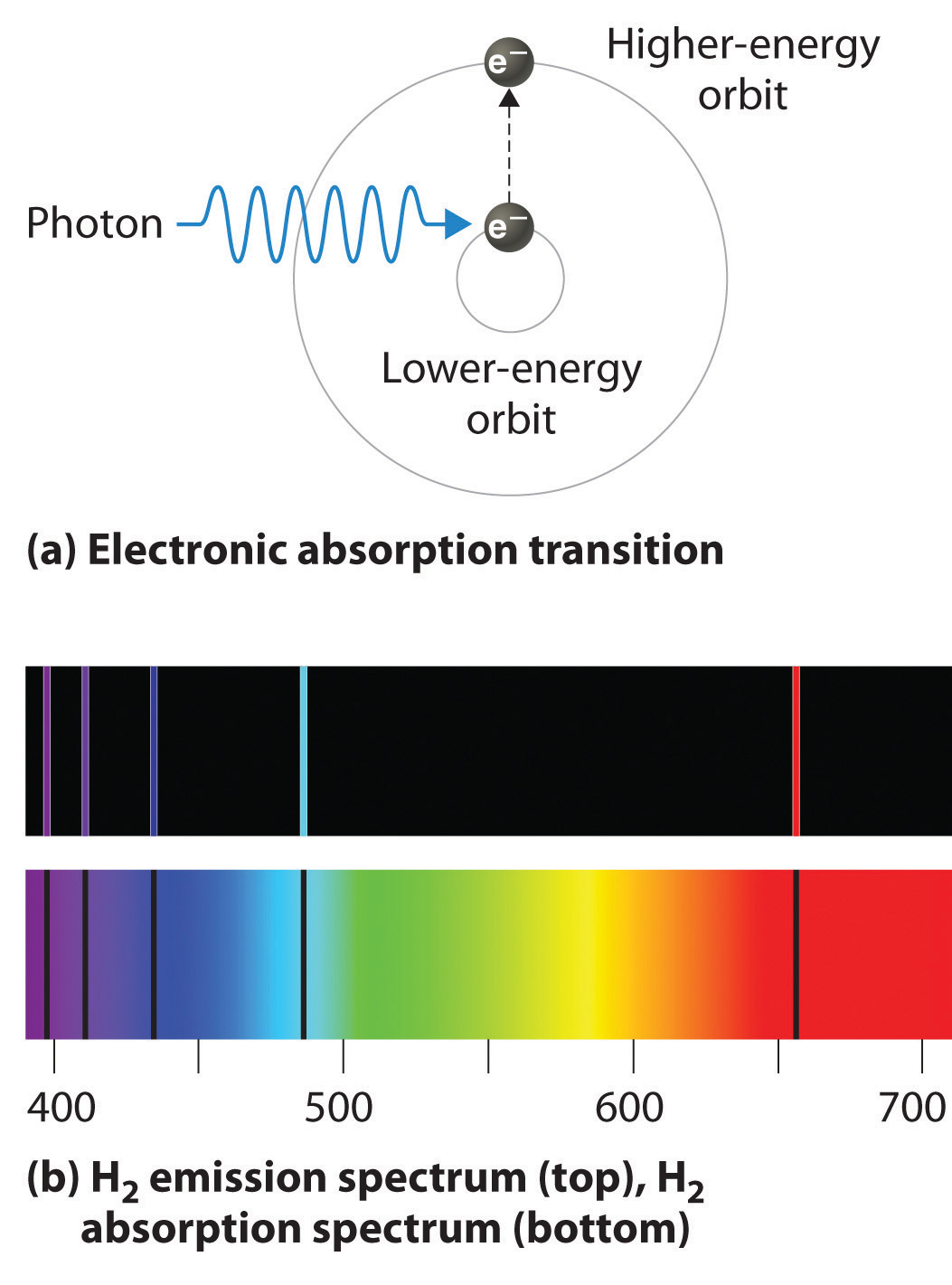
Electronic Spectroscopy Bands . Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength.
from worksheetsufertatstl.z21.web.core.windows.net
Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Electronic spectroscopy relies on the quantized nature of energy states.
Atomic Spectra And Its Types
Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from.
From worksheetsufertatstl.z21.web.core.windows.net
Atomic Spectra And Its Types Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Electronic spectroscopy relies on the quantized nature of energy states. In. Electronic Spectroscopy Bands.
From www.researchgate.net
Optical emission spectra of the OH (AX) band recorded for the... Download Scientific Diagram Electronic Spectroscopy Bands In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which. Electronic Spectroscopy Bands.
From www.researchgate.net
The emission spectrum of N2(CB) band ∆ν = −2 and ∆ν = −3 marked by... Download Scientific Diagram Electronic Spectroscopy Bands A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of. Electronic Spectroscopy Bands.
From www.slideserve.com
PPT Electronic spectra of transition metal complexes PowerPoint Presentation ID270645 Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at. Electronic Spectroscopy Bands.
From www.researchgate.net
1 The entire spectrum highlighting the infrared band... Download Scientific Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy. Electronic Spectroscopy Bands.
From www.researchgate.net
XPS valence band spectra of (a) Pd, (b) Ag, and (c) Cu nanoparticles (D... Download Scientific Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Electronic spectroscopy relies on the quantized nature of. Electronic Spectroscopy Bands.
From chem.libretexts.org
Electronic Spectroscopy Interpretation Chemistry LibreTexts Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy relies on the quantized nature of energy states. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. In. Electronic Spectroscopy Bands.
From flexautomotive.net
EMC FLEX BLOG Spectrum Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. In this chapter, we begin with a discussion of the basic principles of. Electronic Spectroscopy Bands.
From www.researchgate.net
(a) XPS Ag3d spectra, (b) MNN Auger electron spectra, and (c) valence... Download Scientific Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption. Electronic Spectroscopy Bands.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each. Electronic Spectroscopy Bands.
From www.weizmann.ac.il
Electron Spectroscopy (XPS) Band diagrams using CREM Chemical Research Support Electronic Spectroscopy Bands In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption. Electronic Spectroscopy Bands.
From www.researchgate.net
(A) Valence band XPS spectrum of VO2,... Download Scientific Diagram Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the. Electronic Spectroscopy Bands.
From chem.libretexts.org
9.1 The Spectrum Chemistry LibreTexts Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. In this chapter,. Electronic Spectroscopy Bands.
From www.researchgate.net
Porphyrin absorption spectrum. a = Soret band; b = Q band. Download Scientific Diagram Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter,. Electronic Spectroscopy Bands.
From andor.oxinst.com
Introduction to Transient Spectroscopy and its applications Oxford Instruments Electronic Spectroscopy Bands Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at. Electronic Spectroscopy Bands.
From phys.libretexts.org
2.3 The Spectrum Physics LibreTexts Electronic Spectroscopy Bands A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy relies on the quantized nature of. Electronic Spectroscopy Bands.
From chem.libretexts.org
EPR Interpretation Chemistry LibreTexts Electronic Spectroscopy Bands A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy relies on the quantized nature of energy states. In. Electronic Spectroscopy Bands.
From www.researchgate.net
(a) Schematic illustration of band structures and resonant Raman scattering Download Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each. Electronic Spectroscopy Bands.
From www.researchgate.net
7 2D electronic spectroscopy information content in a 2D spectrum. (a)... Download Scientific Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be. Electronic Spectroscopy Bands.
From www.researchgate.net
Electronic structure and spindegenerate bands of bulk MoSe2. (a)... Download Scientific Diagram Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can. Electronic Spectroscopy Bands.
From www.researchgate.net
a) Ultraviolet photoelectron spectroscopy (UPS) spectra. b) Energy band... Download Scientific Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption. Electronic Spectroscopy Bands.
From vdocuments.mx
Electronic band structure of HgSe from Fourier transform spectroscopy [Download PDF] Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy. Electronic Spectroscopy Bands.
From www.researchgate.net
Oxygen absorption bands and electronic transitions in the optical range... Download Scientific Electronic Spectroscopy Bands A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy. Electronic Spectroscopy Bands.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Electronic Spectroscopy Bands In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each. Electronic Spectroscopy Bands.
From www.researchgate.net
Electronic absorption spectra of VTCA copolymer (1), gold 6.8 wt.... Download Scientific Diagram Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at. Electronic Spectroscopy Bands.
From mynewblogsteetburt258.blogspot.com
WHAT IS SPECTRUM ? / EXPLAIN ITS FOLLOWING BANDS Electronic Spectroscopy Bands Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at. Electronic Spectroscopy Bands.
From pubs.acs.org
XBand ParallelMode and Multifrequency Electron Resonance Spectroscopy of S = 1/2 Electronic Spectroscopy Bands Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at. Electronic Spectroscopy Bands.
From www.slideserve.com
PPT Lecture 36 Electronic spectroscopy PowerPoint Presentation, free download ID2077297 Electronic Spectroscopy Bands In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be. Electronic Spectroscopy Bands.
From chem.libretexts.org
Electronic Spectroscopy Interpretation Chemistry LibreTexts Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at. Electronic Spectroscopy Bands.
From www.researchgate.net
Electron resonance (EPR) spectroscopy of {Gd2}. a Xband... Download Scientific Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Electronic spectroscopy relies on the quantized nature of energy states. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. In this chapter, we begin with a discussion of the basic. Electronic Spectroscopy Bands.
From www.slideserve.com
PPT Electron spectroscopy PowerPoint Presentation, free download ID4772440 Electronic Spectroscopy Bands A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited. Electronic Spectroscopy Bands.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Electronic Spectroscopy Bands Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. Given enough energy, an electron can be. Electronic Spectroscopy Bands.
From www.slideserve.com
PPT Electronic band structure of CrN and GdN from xray spectroscopy PowerPoint Presentation Electronic Spectroscopy Bands Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Electronic spectroscopy relies on the quantized nature of energy states. In. Electronic Spectroscopy Bands.
From chem.libretexts.org
Electronic Spectroscopy Interpretation Chemistry LibreTexts Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. In this chapter, we begin with a discussion of the basic principles of the electronic spectroscopy of diatomic molecules.(6,7) the rest of the. A uv/vis spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each. Electronic Spectroscopy Bands.
From www.researchgate.net
UVVis spectrum of the LMCT electronic transition mechanism. Koushik et... Download Scientific Electronic Spectroscopy Bands Given enough energy, an electron can be excited from. Ultraviolet photoelectron spectroscopy corresponds to incident photons in the uv region; Electronic spectroscopy relies on the quantized nature of energy states. Given enough energy, an electron can be excited from. Electronic spectroscopy relies on the quantized nature of energy states. A uv/vis spectrometer records the wavelengths at which absorption occurs, together. Electronic Spectroscopy Bands.