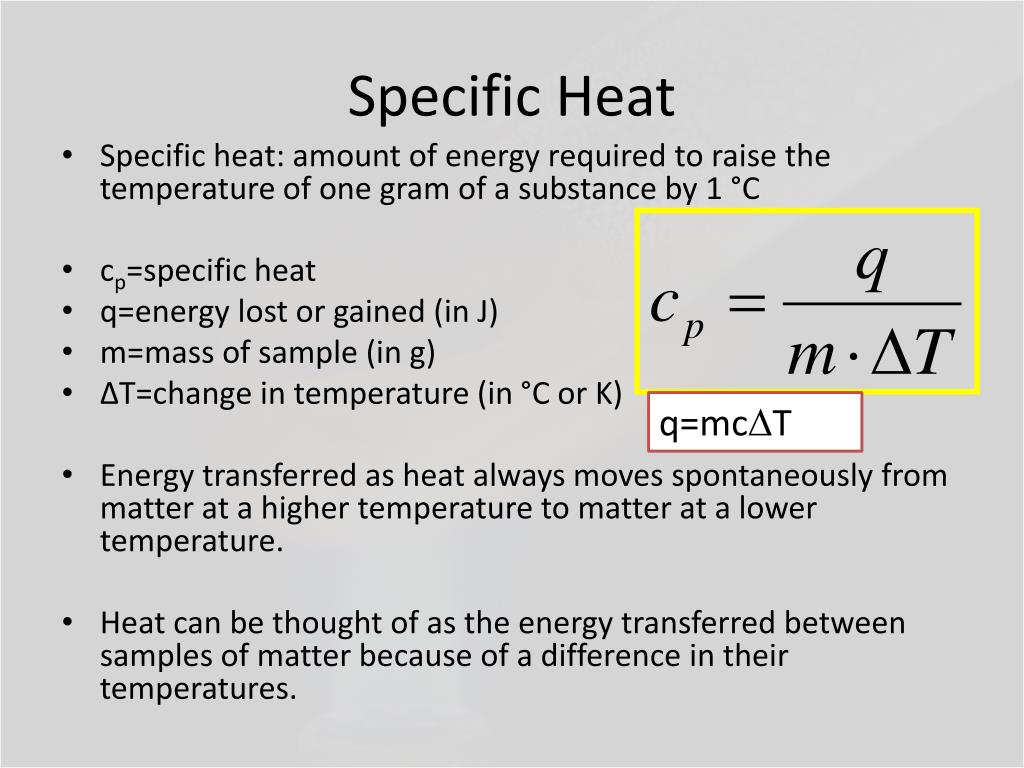
Specific Heat Definition Simple . Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. It plays a crucial role in understanding. Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. The symbol c stands for specific heat and depends on the material and phase. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat is the amount of heat necessary to change the temperature of 1.00. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an.
from www.slideserve.com
The symbol c stands for specific heat and depends on the material and phase. It plays a crucial role in understanding. Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. The specific heat is the amount of heat necessary to change the temperature of 1.00. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an.
PPT Thermochemistry PowerPoint Presentation, free download ID3170116
Specific Heat Definition Simple Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. The symbol c stands for specific heat and depends on the material and phase. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. It plays a crucial role in understanding. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat is the amount of heat necessary to change the temperature of 1.00. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an.
From www.slideserve.com
PPT Heat and Temperature PowerPoint Presentation, free download ID Specific Heat Definition Simple The specific heat is the amount of heat necessary to change the temperature of 1.00. The symbol c stands for specific heat and depends on the material and phase. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature. Specific Heat Definition Simple.
From studylib.net
1.3 Specific Heat Capacity Specific Heat Definition Simple It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature of. Specific Heat Definition Simple.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID2649066 Specific Heat Definition Simple It plays a crucial role in understanding. The symbol c stands for specific heat and depends on the material and phase. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a. Specific Heat Definition Simple.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation ID5733870 Specific Heat Definition Simple Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. It plays a crucial role in understanding. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. Specific heat refers to the ratio of the quantity of heat that we require to. Specific Heat Definition Simple.
From www.youtube.com
Specific heat capacity YouTube Specific Heat Definition Simple Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Everything you need. Specific Heat Definition Simple.
From www.youtube.com
Heat Capacity and Specific Heat YouTube Specific Heat Definition Simple The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat refers to the ratio of the quantity of heat. Specific Heat Definition Simple.
From www.slideserve.com
PPT Specific Heat Capacity and Latent Heat PowerPoint Presentation Specific Heat Definition Simple Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. It plays. Specific Heat Definition Simple.
From www.slideserve.com
PPT Chapter 2 The Energy Cycle PowerPoint Presentation, free download Specific Heat Definition Simple Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. The specific. Specific Heat Definition Simple.
From studylib.net
Heat Equation Specific Heat Definition Simple The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. It plays a crucial role in understanding. The symbol c stands for specific heat and depends on the material and phase. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how. Specific Heat Definition Simple.
From gamesmartz.com
Specific Heat Definition & Image GameSmartz Specific Heat Definition Simple Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. Specific heat is defined as the amount of. Specific Heat Definition Simple.
From education-portal.com
Latent Heat Definition, Formula & Examples Video & Lesson Transcript Specific Heat Definition Simple The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The symbol c stands for specific heat and depends on the. Specific Heat Definition Simple.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Specific Heat Definition Simple Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. It plays a crucial role in understanding. Specific heat refers to the ratio of the quantity of. Specific Heat Definition Simple.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3170116 Specific Heat Definition Simple Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. Everything you. Specific Heat Definition Simple.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources Specific Heat Definition Simple Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Everything you need to know about specific heat capacity, its importance. Specific Heat Definition Simple.
From www.yourdictionary.com
Difference Between Heat and Temperature in Simple Terms YourDictionary Specific Heat Definition Simple Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. The specific heat is the amount of heat necessary to change the temperature of 1.00. Specific heat refers to the ratio of. Specific Heat Definition Simple.
From ar.inspiredpencil.com
Heat Definition Specific Heat Definition Simple Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a. Specific Heat Definition Simple.
From pediaa.com
Difference Between Latent Heat and Sensible Heat Definition Specific Heat Definition Simple It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The symbol c stands for specific heat and depends on the material and phase. Specific heat is the amount of heat energy required to raise the temperature of. Specific Heat Definition Simple.
From abramsrewing.blogspot.com
Specific Heat Capacity Definition AbramsrEwing Specific Heat Definition Simple It plays a crucial role in understanding. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. The specific heat is the amount of heat necessary to change the temperature of 1.00. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram. Specific Heat Definition Simple.
From www.slideserve.com
PPT Properties of Water Notes PowerPoint Presentation, free download Specific Heat Definition Simple It plays a crucial role in understanding. Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat refers to the ratio of the quantity. Specific Heat Definition Simple.
From www.slideserve.com
PPT Temperature, Thermal Energy, and Heat PowerPoint Presentation Specific Heat Definition Simple Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by. Specific Heat Definition Simple.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Specific Heat Definition Simple Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. It plays a crucial role in understanding. Specific. Specific Heat Definition Simple.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Specific Heat Definition Simple The symbol c stands for specific heat and depends on the material and phase. It plays a crucial role in understanding. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Specific heat is the amount of heat energy required to raise the temperature of a. Specific Heat Definition Simple.
From edumentors.co.uk
GCSE Physics Particle model of matter Edumentors Specific Heat Definition Simple Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. The symbol c stands for specific heat and. Specific Heat Definition Simple.
From study.com
Heat of Fusion Definition, Equation & Examples Video & Lesson Specific Heat Definition Simple Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat is the amount of heat necessary to change the temperature of 1.00. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of. Specific Heat Definition Simple.
From www.downwindkennels.com
Specific Heat Capacity Specific Heat Definition Simple It plays a crucial role in understanding. The symbol c stands for specific heat and depends on the material and phase. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. Everything you need to know about. Specific Heat Definition Simple.
From www.slideshare.net
Specific Heat Specific Heat Definition Simple The specific heat is the amount of heat necessary to change the temperature of 1.00. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The symbol c stands for specific heat and depends on the material and phase. Specific heat is the amount of. Specific Heat Definition Simple.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID1769903 Specific Heat Definition Simple It plays a crucial role in understanding. The symbol c stands for specific heat and depends on the material and phase. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. The specific heat of a substance. Specific Heat Definition Simple.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity Specific Heat Definition Simple The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. It plays a crucial role in understanding. Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount of heat required. Specific Heat Definition Simple.
From www.toppr.com
Specific Heat Formula Definition, Equations, Examples Specific Heat Definition Simple The specific heat is the amount of heat necessary to change the temperature of 1.00. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. Specific heat is defined as the amount of heat required to raise. Specific Heat Definition Simple.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity Specific Heat Definition Simple Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to. Specific Heat Definition Simple.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. Specific Heat Definition Simple The specific heat is the amount of heat necessary to change the temperature of 1.00. Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It. Specific Heat Definition Simple.
From www.youtube.com
Specific Heat Definition What is Specific Heat Capacity Concept of Specific Heat Definition Simple Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. The specific heat is the amount of heat necessary to change. Specific Heat Definition Simple.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video Specific Heat Definition Simple The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. The symbol c stands. Specific Heat Definition Simple.
From www.worksheetsplanet.com
What is Specific Heat Specific Heat Definition Simple Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat. The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1oc. The symbol c stands for specific heat and depends on the material and phase. Specific heat is. Specific Heat Definition Simple.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2249912 Specific Heat Definition Simple It plays a crucial role in understanding. Specific heat is the amount of heat energy required to raise the temperature of a substance by one degree. Specific heat refers to the ratio of the quantity of heat that we require to raise the temperature of a body by one degree that we need to increase the temperature of an. The. Specific Heat Definition Simple.