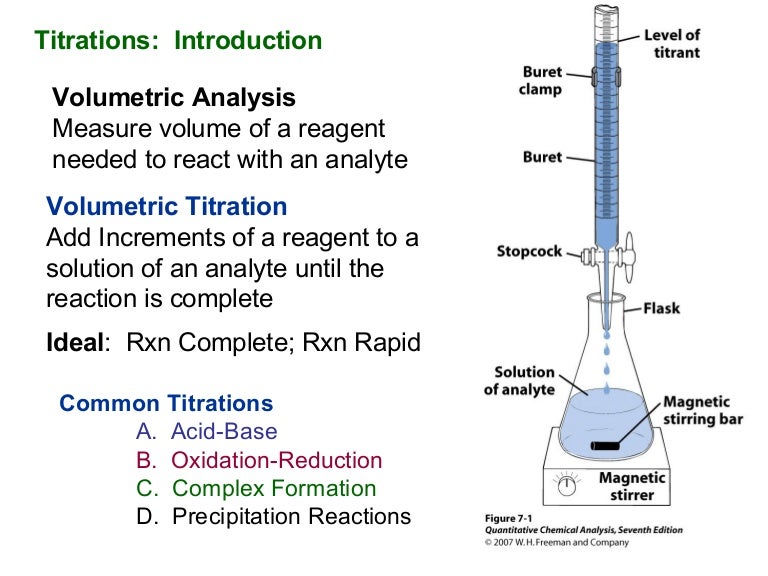
Titration Slideshare . A neutralization reaction is carried. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. It defines titration as a quantitative analytical method. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: The equation to use is: Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. 344 views • 9 slides ch. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. The document provides an overview of titration including terminology, basic concepts, and types of titrations. 4 steps to solve titration. Titration is used specifically when you have an unknown concentration of an acid or base. The solution with known concentration delivered into the “unknown” •. Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by.
from www.slideshare.net
A neutralization reaction is carried. It defines titration as a quantitative analytical method. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. The solution with known concentration delivered into the “unknown” •. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: 4 steps to solve titration. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost.
Intro to titrations
Titration Slideshare The equation to use is: Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. The equation to use is: It defines titration as a quantitative analytical method. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: The solution with known concentration delivered into the “unknown” •. 4 steps to solve titration. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. 344 views • 9 slides ch. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. A neutralization reaction is carried. Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. Titration is used specifically when you have an unknown concentration of an acid or base.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation ID2145680 Titration Slideshare Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. The document provides an overview of titration including terminology, basic concepts, and types of titrations. 4 steps to solve titration. It defines titration as a quantitative analytical method. Acid/base titration technique used to determine the concentration of an acid or base by. Titration Slideshare.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration Slideshare Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: 4 steps to solve titration. Titration is used specifically when you have an unknown concentration of an acid or base. It defines titration as. Titration Slideshare.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Slideshare Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. The equation to use is: 4 steps to solve titration. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. Acid/base titration technique used to determine the concentration. Titration Slideshare.
From jagomart.net
Titration Procedure Pdf 88357 Lab Virtual Titration Titration Slideshare 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: A neutralization reaction is carried. 344 views • 9 slides ch. Titrations in. Titration Slideshare.
From www.slideshare.net
Chemical calculations part 3 titrations Titration Slideshare Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. Titrations in a titration a solution of accurately known. Titration Slideshare.
From www.slideshare.net
Precipitation titrations Titration Slideshare Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. 4 steps to solve titration. The solution with known concentration delivered into the “unknown” •. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. It defines titration as a quantitative analytical method. Titration. Titration Slideshare.
From www.savemyexams.co.uk
Titrations (1.7.2) Edexcel A Level Chemistry Revision Notes 2017 Titration Slideshare The equation to use is: Titration is used specifically when you have an unknown concentration of an acid or base. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision,. Titration Slideshare.
From www.slideshare.net
Acid base titration Titration Slideshare Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. Titration is used specifically when you have an unknown concentration of an acid or base. The document provides an overview of titration including terminology,. Titration Slideshare.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Titration Slideshare 344 views • 9 slides ch. A neutralization reaction is carried. Titration is used specifically when you have an unknown concentration of an acid or base. The solution with known concentration delivered into the “unknown” •. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. 4 steps to solve titration. The. Titration Slideshare.
From www.slideshare.net
Potassium permanganate titrations Titration Slideshare Titration is used specifically when you have an unknown concentration of an acid or base. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Titration, also known as titrimetry, is a technique used to determine the. Titration Slideshare.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID3459790 Titration Slideshare Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. 344 views • 9 slides ch. It defines titration as a quantitative analytical method. Titration is used in various fields such as agriculture, oil. Titration Slideshare.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Titration Slideshare 344 views • 9 slides ch. Titration is used specifically when you have an unknown concentration of an acid or base. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Titration, also known as titrimetry, is. Titration Slideshare.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Slideshare 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. Titrations in a titration a solution of accurately known concentration is added gradually. Titration Slideshare.
From ar.inspiredpencil.com
Titration Diagram Titration Slideshare 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. It defines titration as a quantitative analytical method. A neutralization reaction is carried. The document provides an overview of titration including terminology, basic concepts, and. Titration Slideshare.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Slideshare Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. The document provides an overview of titration including terminology, basic concepts, and types of titrations. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Titration is used specifically when you have an unknown concentration. Titration Slideshare.
From www.slideshare.net
Acid base titration Titration Slideshare The document provides an overview of titration including terminology, basic concepts, and types of titrations. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. A neutralization reaction is carried. The equation to use is: 4 steps to solve titration. 344 views • 9 slides ch. Titration, also known as titrimetry, is. Titration Slideshare.
From mavink.com
Titration Color Titration Slideshare Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. Titration is used specifically when you have an unknown concentration of an acid or base. A neutralization reaction is carried. The solution with known concentration delivered into the “unknown” •. 344 views • 9 slides ch.. Titration Slideshare.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Slideshare It defines titration as a quantitative analytical method. 344 views • 9 slides ch. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. The equation to use is: Titration • titrations are used to. Titration Slideshare.
From www.slideshare.net
Neutralization titrations Titration Slideshare It defines titration as a quantitative analytical method. A neutralization reaction is carried. 4 steps to solve titration. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 =. Titration Slideshare.
From www.slideshare.net
Intro to titrations Titration Slideshare Titration is used specifically when you have an unknown concentration of an acid or base. It defines titration as a quantitative analytical method. The solution with known concentration delivered into the “unknown” •. A neutralization reaction is carried. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision,. Titration Slideshare.
From www.slideshare.net
Acid base titration Titration Slideshare A neutralization reaction is carried. The equation to use is: The document provides an overview of titration including terminology, basic concepts, and types of titrations. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. It defines titration as a quantitative analytical method. Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown. Titration Slideshare.
From www.learnsci.com
LearnSci LabSim Titration End Point Titration Slideshare The equation to use is: Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. 4 steps to solve titration. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. A neutralization reaction is carried. The document provides an overview of titration including terminology, basic concepts, and types of titrations. The. Titration Slideshare.
From www.sliderbase.com
Titration Titration Slideshare Titration • titrations are used to determine the amount of acid or base in a solution • titrant: Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Titrations in a. Titration Slideshare.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Slideshare A neutralization reaction is carried. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. 344 views • 9 slides ch. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. It defines titration as a quantitative analytical. Titration Slideshare.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Slideshare Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. The equation to use is: Titration • titrations are used to determine the amount of acid or base in a solution • titrant: 344 views • 9 slides ch. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. The document. Titration Slideshare.
From www.youtube.com
Acid Base Titration Lecture 3 Chemistry Practicals Fsc 1st Year Titration Slideshare 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. Titration, also known as titrimetry, is a technique used to determine the concentration. Titration Slideshare.
From amaryllids.ru
IODOMETRIC AND IODIMETRIC TITRATION PDF Titration Slideshare Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Titration is used specifically when you have an unknown concentration of an acid or base. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻). Titration Slideshare.
From www.reagent.co.uk
Who Invented Titration? The Science Blog Titration Slideshare Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. The equation to use is: Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Titrations in a titration a solution of accurately. Titration Slideshare.
From jagomart.net
Aspirin Tablets Titration Titration Slideshare 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. Acid/base titration technique used to determine the. Titration Slideshare.
From www.slideshare.net
karl fischer titration slide Titration Slideshare Titration is used in various fields such as agriculture, oil industry, chemical industry, pharmaceuticals, and food industry due to its accuracy, precision, and cost. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard.. Titration Slideshare.
From www.youtube.com
Precipitation Titrations YouTube Titration Slideshare It defines titration as a quantitative analytical method. 344 views • 9 slides ch. The solution with known concentration delivered into the “unknown” •. A neutralization reaction is carried. Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. The document provides an overview of titration including terminology, basic concepts, and types. Titration Slideshare.
From www.slideshare.net
Complexometric titrations with EDTA Titration Slideshare Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. A neutralization reaction is carried. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Titration • titrations are used to determine the amount of acid or base in a solution • titrant: Titration is used specifically. Titration Slideshare.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Titration Slideshare Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. The solution with known concentration delivered into the “unknown” •. The equation to use is: 344 views • 9 slides ch. 4 steps to solve titration. Titration • titrations are used. Titration Slideshare.
From jagomart.net
Titration Procedure Pdf 89815 Titration Introduction Titration Slideshare Titration, also known as titrimetry, is a technique used to determine the concentration of an unknown solution by. 4 steps to solve titration. 𝑀 𝑎 (#𝐻) 𝑉 𝑎 = 𝑀 𝑏 (#𝑂𝐻) 𝑉 𝑏. The equation to use is: Titration is used specifically when you have an unknown concentration of an acid or base. Titrations in a titration a solution. Titration Slideshare.
From www.slideshare.net
Complexometric titration Titration Slideshare Acid/base titration technique used to determine the concentration of an acid or base by comparison with a standard. 344 views • 9 slides ch. The document provides an overview of titration including terminology, basic concepts, and types of titrations. Titrations in a titration a solution of accurately known concentration is added gradually added to another solution of. Titration, also known. Titration Slideshare.