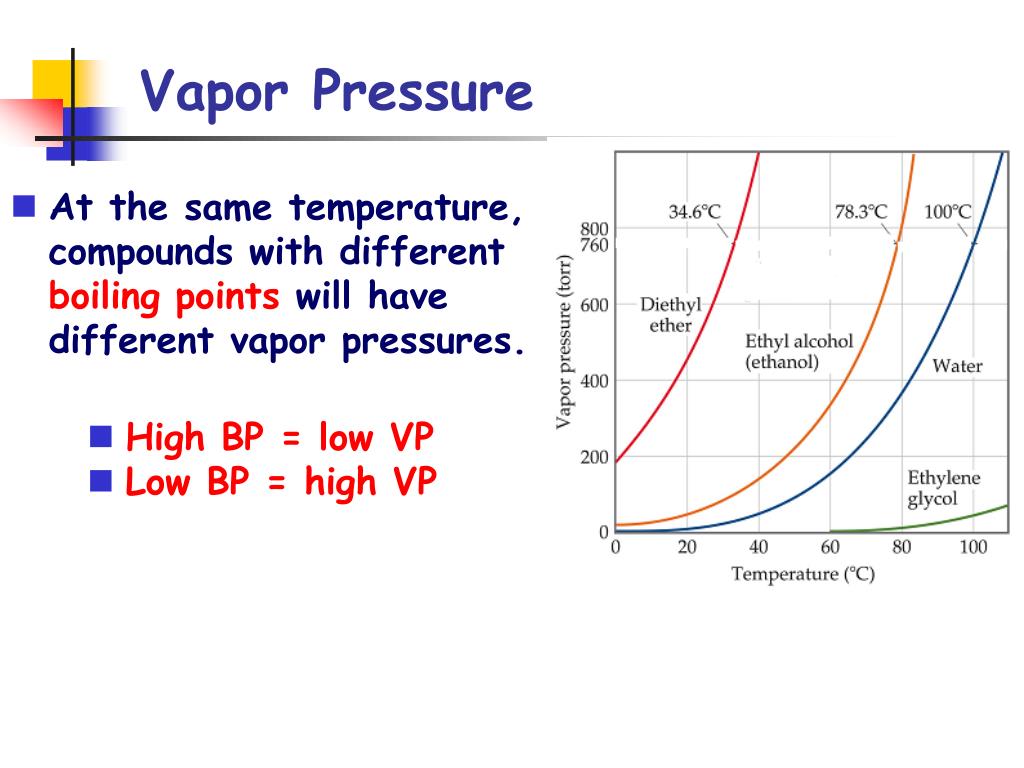
What Is Vapor Pressure And Why Does It Change With Temperature . The pressure scale (the vertical one) is measured in kilopascals (kpa). Factors that affect vapor pressure surface. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Vapor pressure is created by faster molecules that. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2:
from www.slideserve.com
Factors that affect vapor pressure surface. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Vapor pressure is created by faster molecules that. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Describe the relationship between the intermolecular forces in a liquid and its vapor. The pressure scale (the vertical one) is measured in kilopascals (kpa). The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure.
PPT Phase Changes PowerPoint Presentation, free download ID2437650
What Is Vapor Pressure And Why Does It Change With Temperature Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapor pressure is created by faster molecules that. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Describe the relationship between the intermolecular forces in a liquid and its vapor. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Factors that affect vapor pressure surface. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2:
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is created by faster molecules that. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Describe the relationship between the intermolecular forces in a liquid and its vapor. The graph shows how the saturated vapor pressure (svp) of. What Is Vapor Pressure And Why Does It Change With Temperature.
From saylordotorg.github.io
Vapor Pressure What Is Vapor Pressure And Why Does It Change With Temperature The pressure scale (the vertical one) is measured in kilopascals (kpa). Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor What Is Vapor Pressure And Why Does It Change With Temperature Factors that affect vapor pressure surface. Vapor pressure is created by faster molecules that. The pressure scale (the vertical one) is measured in kilopascals (kpa). Describe the relationship between the intermolecular forces in a liquid and its vapor. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is. What Is Vapor Pressure And Why Does It Change With Temperature.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility What Is Vapor Pressure And Why Does It Change With Temperature The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressure is created by faster molecules that. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Describe the relationship between the intermolecular forces. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure And Why Does It Change With Temperature The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapor pressure is created by faster molecules that. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo What Is Vapor Pressure And Why Does It Change With Temperature The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressure is created by. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint What Is Vapor Pressure And Why Does It Change With Temperature The pressure scale (the vertical one) is measured in kilopascals (kpa). Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The pressure exerted by the gas in equilibrium with a solid. What Is Vapor Pressure And Why Does It Change With Temperature.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The pressure scale (the vertical one) is measured in kilopascals (kpa). The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Is Vapor Pressure And Why Does It Change With Temperature Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is. What Is Vapor Pressure And Why Does It Change With Temperature.
From mavink.com
Vapor Pressure And Boiling Point Relationship What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is created by faster molecules that. Factors that affect vapor pressure surface. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Describe the relationship between the. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure And Why Does It Change With Temperature Factors that affect vapor pressure surface. Vapor pressure is created by faster molecules that. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature. What Is Vapor Pressure And Why Does It Change With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel What Is Vapor Pressure And Why Does It Change With Temperature Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressure is created by faster molecules that. Vapor pressures have. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Describe the relationship between the intermolecular forces in a liquid and its vapor. Factors that affect vapor pressure surface. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressure is defined as the pressure at which a gas coexists with its. What Is Vapor Pressure And Why Does It Change With Temperature.
From socratic.org
How does atmospheric pressure change with altitude? Socratic What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is created by faster molecules that. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Describe the relationship between the intermolecular forces in a liquid and its vapor. The pressure exerted. What Is Vapor Pressure And Why Does It Change With Temperature.
From socratic.org
How do mountains affect temperature on both the windward side and the What Is Vapor Pressure And Why Does It Change With Temperature The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Vapor pressure is created by faster molecules that. The pressure exerted by the gas in. What Is Vapor Pressure And Why Does It Change With Temperature.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure And Why Does It Change With Temperature The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Factors that affect vapor pressure surface. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapour pressure, also known as vapour equilibrium pressure, can be defined. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.researchgate.net
The saturation curve of pressure against temperature for chlorine What Is Vapor Pressure And Why Does It Change With Temperature Factors that affect vapor pressure surface. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The graph. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is Vapor Pressure And Why Does It Change With Temperature The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressure is created by faster molecules that. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted. What Is Vapor Pressure And Why Does It Change With Temperature.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws What Is Vapor Pressure And Why Does It Change With Temperature The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a. What Is Vapor Pressure And Why Does It Change With Temperature.
From mavink.com
Water Vapor Pressure Vs Temperature What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Describe the relationship between the intermolecular forces in a liquid and its vapor. The graph shows how the. What Is Vapor Pressure And Why Does It Change With Temperature.
From slideplayer.com
Unit 9 Gases. ppt download What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is created by faster molecules that. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Describe the relationship between the intermolecular forces in a liquid. What Is Vapor Pressure And Why Does It Change With Temperature.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is created by faster molecules that. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by. What Is Vapor Pressure And Why Does It Change With Temperature.
From mungfali.com
Water Vapor Pressure Temperature Chart What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is Vapor Pressure And Why Does It Change With Temperature Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Describe the relationship between the intermolecular forces in a liquid and its vapor. The pressure exerted by the gas. What Is Vapor Pressure And Why Does It Change With Temperature.
From socratic.org
What is the relation between critical temperature and boiling point or What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapor pressure is created by faster molecules that. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation ID352441 What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Describe the relationship between the intermolecular forces in a liquid and its vapor. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapor pressure is defined as. What Is Vapor Pressure And Why Does It Change With Temperature.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is created by faster molecules that. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapor. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Is Vapor Pressure And Why Does It Change With Temperature The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapor pressure is created by faster molecules that. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure. What Is Vapor Pressure And Why Does It Change With Temperature.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of What Is Vapor Pressure And Why Does It Change With Temperature Factors that affect vapor pressure surface. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The pressure scale. What Is Vapor Pressure And Why Does It Change With Temperature.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapor Pressure And Why Does It Change With Temperature The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapor pressure is created by faster molecules that. The pressure scale (the vertical one) is measured in kilopascals (kpa). Factors that affect vapor pressure surface. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapour pressure,. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.youtube.com
Which Compound Has a Higher Vapor Pressure? Intermolecular Force What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Factors that affect vapor pressure surface. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapor pressure is created by faster molecules that. Vapour pressure, also known as vapour equilibrium pressure, can be defined as. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co What Is Vapor Pressure And Why Does It Change With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Describe the relationship between the intermolecular forces in a liquid and its vapor. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Vapour pressure, also known as. What Is Vapor Pressure And Why Does It Change With Temperature.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts What Is Vapor Pressure And Why Does It Change With Temperature Factors that affect vapor pressure surface. The pressure scale (the vertical one) is measured in kilopascals (kpa). The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. Vapor pressures have an exponential relationship with temperature. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure And Why Does It Change With Temperature Describe the relationship between the intermolecular forces in a liquid and its vapor. Vapor pressure is created by faster molecules that. Factors that affect vapor pressure surface. Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. The pressure scale (the vertical one) is measured in kilopascals (kpa). Vapor pressures have an. What Is Vapor Pressure And Why Does It Change With Temperature.
From www.numerade.com
From the plot of vapor pressures vs temperature above, estimate the What Is Vapor Pressure And Why Does It Change With Temperature Factors that affect vapor pressure surface. Describe the relationship between the intermolecular forces in a liquid and its vapor. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour. What Is Vapor Pressure And Why Does It Change With Temperature.