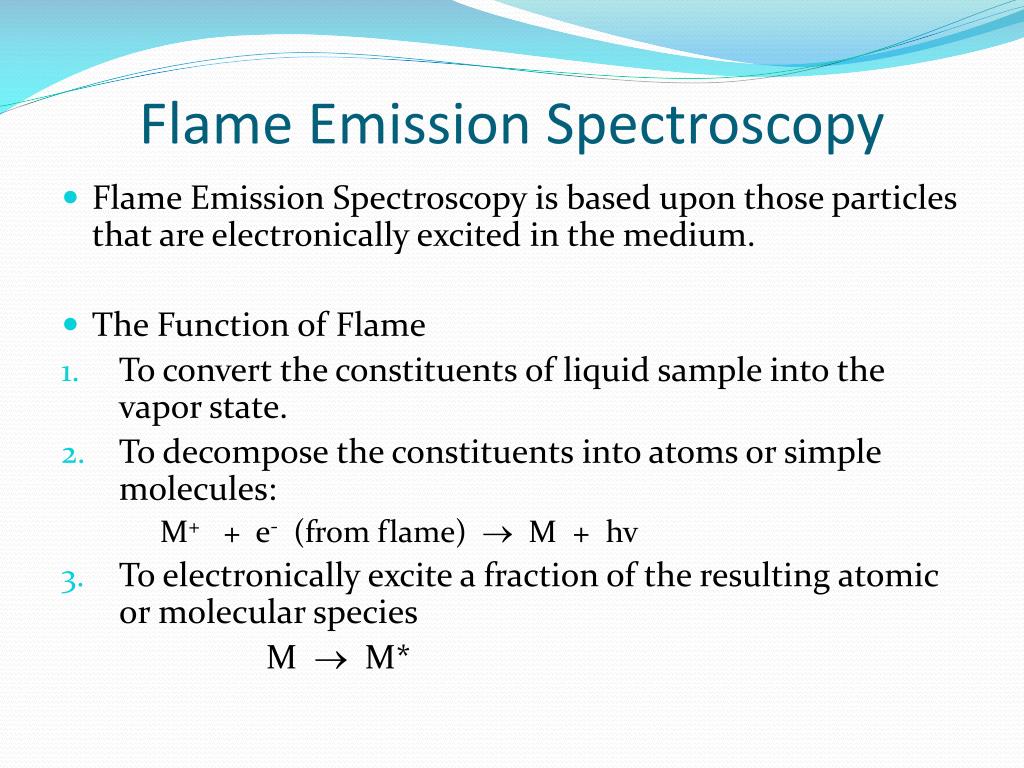
Flame Spectroscopy Slideshare . The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Plasmas produce higher atomization ratios, but. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Atoms are excited by thermal collisions in the flame. Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. What is flame emission spectroscopy? Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a.
from www.slideserve.com
What is flame emission spectroscopy? Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Atoms are excited by thermal collisions in the flame. Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Plasmas produce higher atomization ratios, but. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark.
PPT Atomic Emission Spectrometry PowerPoint Presentation, free
Flame Spectroscopy Slideshare Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Plasmas produce higher atomization ratios, but. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Atoms are excited by thermal collisions in the flame. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. What is flame emission spectroscopy?
From www.slideserve.com
PPT Flame Spectroscopy PowerPoint Presentation, free download ID Flame Spectroscopy Slideshare Atoms are excited by thermal collisions in the flame. What is flame emission spectroscopy? Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Plasmas produce higher atomization ratios, but. Flame spectroscopy. Flame Spectroscopy Slideshare.
From pt.slideshare.net
Flame Atomic Absorption Spectroscopy Flame Spectroscopy Slideshare Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths. Flame Spectroscopy Slideshare.
From knowledge.carolina.com
Flame Tests and Spectroscopy Get Excited About Color Carolina Flame Spectroscopy Slideshare Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Atoms are excited by thermal collisions in the flame. Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. What is flame emission. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame photometry Flame Spectroscopy Slideshare Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. What is flame emission spectroscopy? Flame atomic emission spectroscopy (faes) is a classical. Flame Spectroscopy Slideshare.
From www.youtube.com
Flame Atomic Absorption Spectroscopy Demonstration YouTube Flame Spectroscopy Slideshare The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. What is flame emission spectroscopy? Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Atoms are excited. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy Flame Spectroscopy Slideshare Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Atoms are excited by thermal collisions in the flame. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Flame emission spectroscopy involves. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame photometer (Atomic Emission Spectroscopy) Flame emission spectr… Flame Spectroscopy Slideshare Atoms are excited by thermal collisions in the flame. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Plasmas produce higher atomization ratios, but. What is flame emission spectroscopy? The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame emission. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame Atomic Absorption Spectroscopy Flame Spectroscopy Slideshare Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. What. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy Flame Spectroscopy Slideshare Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame emission spectroscopy provides us atomic emission spectrum. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission & atomic absorption spectroscopy Flame Spectroscopy Slideshare Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Plasmas produce higher atomization ratios, but. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame Atomic Absorption Spectroscopy Flame Spectroscopy Slideshare Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame emission spectroscopy measures the light emitted from excited atoms in a flame,. Flame Spectroscopy Slideshare.
From de.slideshare.net
Flame emission spectroscopy and atomic absorption spectroscopy ppt Flame Spectroscopy Slideshare Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. The experiment. Flame Spectroscopy Slideshare.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission Flame Spectroscopy Slideshare Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Atoms are excited by thermal collisions in the. Flame Spectroscopy Slideshare.
From www.studypool.com
SOLUTION Flame emission spectroscopy Studypool Flame Spectroscopy Slideshare Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Flame atomic emission spectroscopy (faes) is a classical method which has been. Flame Spectroscopy Slideshare.
From www.slideserve.com
PPT Flame Photometry F lame atomic emission spectrometry PowerPoint Flame Spectroscopy Slideshare Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc,. Flame Spectroscopy Slideshare.
From mavink.com
Flame Emission Spectroscopy Diagram Flame Spectroscopy Slideshare Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. What is flame emission spectroscopy? Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. The experiment conducted takes advantage of prisms, and their ability to break light up. Flame Spectroscopy Slideshare.
From www.savemyexams.com
Flame Emission Spectroscopy AQA GCSE Chemistry Revision Notes 2018 Flame Spectroscopy Slideshare Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Plasmas produce higher atomization ratios, but. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame photometry Flame Spectroscopy Slideshare Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Atoms are excited by thermal collisions in the flame. Plasmas produce higher atomization ratios, but. Flame emission spectroscopy measures the light emitted from excited atoms. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy and atomic absorption spectroscopy ppt Flame Spectroscopy Slideshare Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting. Flame Spectroscopy Slideshare.
From www.slideserve.com
PPT FLAME SPECTROSCOPY PowerPoint Presentation, free download ID Flame Spectroscopy Slideshare The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. Flame emission. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy and atomic absorption spectroscopy ppt Flame Spectroscopy Slideshare The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. What is flame emission spectroscopy? Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Atoms are excited by thermal collisions in the flame. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy Flame Spectroscopy Slideshare Atoms are excited by thermal collisions in the flame. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy Flame Spectroscopy Slideshare Plasmas produce higher atomization ratios, but. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. Flame emission spectroscopy involves nebulizing a sample solution into. Flame Spectroscopy Slideshare.
From namrataheda.blogspot.com
B for Biology Spectrophotometry Flame Photometry Flame Spectroscopy Slideshare Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. Plasmas produce higher atomization ratios, but. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Flame atomic emission spectroscopy (faes) is a. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame Atomic Absorption Spectroscopy Flame Spectroscopy Slideshare Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Atoms are excited by thermal collisions in the flame. Flame emission spectroscopy involves nebulizing a. Flame Spectroscopy Slideshare.
From www.youtube.com
Atomic Spectroscopy Flame Emission The Flame Test YouTube Flame Spectroscopy Slideshare Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Atoms are excited by thermal. Flame Spectroscopy Slideshare.
From evulpo.com
Flame emission spectroscopy Chemistry Explanation & Exercises evulpo Flame Spectroscopy Slideshare The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Plasmas produce higher atomization ratios, but. Atoms are. Flame Spectroscopy Slideshare.
From www.youtube.com
Flame Photometry Flame Photometer Atomic Emission Spectroscopy Flame Spectroscopy Slideshare Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Plasmas produce higher atomization ratios, but. Flame emission spectroscopy involves nebulizing a sample. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy Flame Spectroscopy Slideshare What is flame emission spectroscopy? Plasmas produce higher atomization ratios, but. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Atomic. Flame Spectroscopy Slideshare.
From www.slideshare.net
Flame emission spectroscopy PPT Flame Spectroscopy Slideshare Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Flame emission spectroscopy involves nebulizing a sample solution into a flame where it is vaporized and atomized. What is flame emission spectroscopy? The experiment conducted takes advantage of prisms, and their ability to break light up into the individual.. Flame Spectroscopy Slideshare.
From www.slideserve.com
PPT Atomic Emission Spectrometry PowerPoint Presentation, free Flame Spectroscopy Slideshare Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Flame photometry is a technique for elemental analysis that uses the characteristic wavelengths of light emitted from atoms. Flame atomic emission spectroscopy (faes) is a classical. Flame Spectroscopy Slideshare.
From www.slideserve.com
PPT Flame Spectroscopy Lab PowerPoint Presentation, free download Flame Spectroscopy Slideshare Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark. What is flame emission spectroscopy? Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while. Flame Spectroscopy Slideshare.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Flame Spectroscopy Slideshare Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Flame spectroscopy • the method uses flame excitation • atoms are exited from the heat of the flame to emit light • exited atoms glow, emitting light at various wavelengths. Flame atomic emission spectroscopy (faes) is a classical method which has been largely. Flame Spectroscopy Slideshare.
From www.youtube.com
ATOMIC EMISSION SPECTROSCOPY Flame Photometry Instrumentation Flame Spectroscopy Slideshare The experiment conducted takes advantage of prisms, and their ability to break light up into the individual. Plasmas produce higher atomization ratios, but. Flame emission spectroscopy measures the light emitted from excited atoms in a flame, while atomic absorption spectroscopy. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. Flame Spectroscopy Slideshare.
From mavink.com
Flame Emission Spectroscopy Diagram Flame Spectroscopy Slideshare Atoms are excited by thermal collisions in the flame. Flame emission spectroscopy provides us atomic emission spectrum based on the excitation of atoms from a lower energy state to a. Flame atomic emission spectroscopy (faes) is a classical method which has been largely displaced by plasma spectroscopies. Atomic emission spectroscopy (aes) is a method of chemical analysis that uses the. Flame Spectroscopy Slideshare.