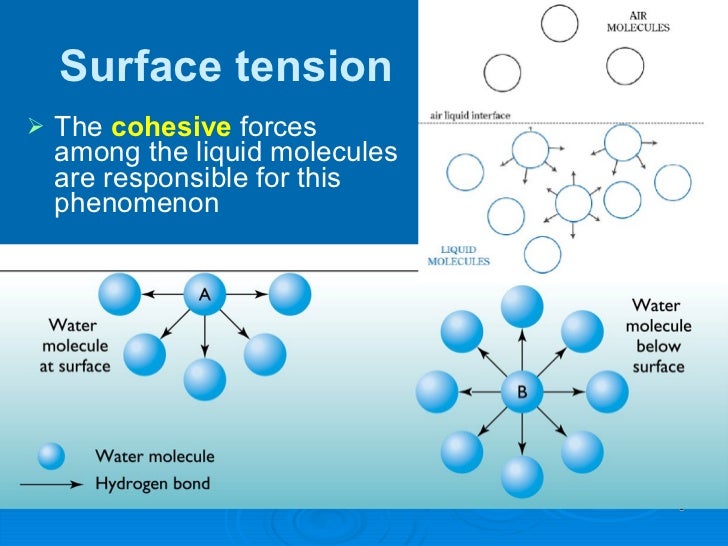
How To Break Surface Tension Of Water . These clusters also help reduce the surface tension of. The surface tension of the water must be broken for sufficient gas exchange. As a result, objects floating in water will sink or change shape as. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Fortunately, creating surface agitation is easily. Detergent molecules' two ends make it able to break through the surface tension of water. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. The surface tension of water bridges the pores in the finely woven material. The end of the detergent molecule which attaches to fat (grease) repels. However, touching the tent will break the surface tension, seeping water through the tent. Since these intermolecular forces vary. Surfactants such as dish soap break up water’s surface tension. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces.
from www.slideshare.net
Since these intermolecular forces vary. These clusters also help reduce the surface tension of. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. The surface tension of water bridges the pores in the finely woven material. However, touching the tent will break the surface tension, seeping water through the tent. As a result, objects floating in water will sink or change shape as. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. The surface tension of the water must be broken for sufficient gas exchange. Detergent molecules' two ends make it able to break through the surface tension of water.
Measuring the Surface Tension of Water by Light Diffraction on Capill…
How To Break Surface Tension Of Water The surface tension of the water must be broken for sufficient gas exchange. These clusters also help reduce the surface tension of. Surfactants such as dish soap break up water’s surface tension. Detergent molecules' two ends make it able to break through the surface tension of water. However, touching the tent will break the surface tension, seeping water through the tent. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. Since these intermolecular forces vary. Fortunately, creating surface agitation is easily. The surface tension of the water must be broken for sufficient gas exchange. The surface tension of water bridges the pores in the finely woven material. The end of the detergent molecule which attaches to fat (grease) repels. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. As a result, objects floating in water will sink or change shape as. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 How To Break Surface Tension Of Water Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. Since these intermolecular forces vary. As a result, objects floating in water will sink or change shape as. Fortunately, creating surface agitation is easily. Your goal is to reduce the surface tension of the water so that it does not support the formation of large. How To Break Surface Tension Of Water.
From lexica.art
Lexica Breaking the surface tension of water, photograph How To Break Surface Tension Of Water Detergent molecules' two ends make it able to break through the surface tension of water. The surface tension of water bridges the pores in the finely woven material. Surfactants such as dish soap break up water’s surface tension. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break. How To Break Surface Tension Of Water.
From errantscience.com
ErrantScience Water surface tension How To Break Surface Tension Of Water Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. Since these intermolecular forces vary. The end of the detergent molecule which attaches to fat (grease) repels. However, touching the tent will break the surface tension, seeping water through the tent. These clusters also help. How To Break Surface Tension Of Water.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download How To Break Surface Tension Of Water Fortunately, creating surface agitation is easily. These clusters also help reduce the surface tension of. The surface tension of the water must be broken for sufficient gas exchange. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. Since these intermolecular forces vary. The surface. How To Break Surface Tension Of Water.
From byjus.com
Explain the surface tension phenomenon with examples. How To Break Surface Tension Of Water Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. Detergent molecules' two ends make it able to break through the surface tension of water. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object. How To Break Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How To Break Surface Tension Of Water However, touching the tent will break the surface tension, seeping water through the tent. As a result, objects floating in water will sink or change shape as. These clusters also help reduce the surface tension of. Since these intermolecular forces vary. Fortunately, creating surface agitation is easily. Due to the surface tension, small objects will float on the surface of. How To Break Surface Tension Of Water.
From www.youtube.com
Surface Tension of Water Explained YouTube How To Break Surface Tension Of Water The surface tension of water bridges the pores in the finely woven material. Since these intermolecular forces vary. These clusters also help reduce the surface tension of. The surface tension of the water must be broken for sufficient gas exchange. Surfactants such as dish soap break up water’s surface tension. As a result, objects floating in water will sink or. How To Break Surface Tension Of Water.
From errantscience.com
Water surface tension ErrantScience How To Break Surface Tension Of Water These clusters also help reduce the surface tension of. However, touching the tent will break the surface tension, seeping water through the tent. Detergent molecules' two ends make it able to break through the surface tension of water. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. Your goal is to reduce the surface. How To Break Surface Tension Of Water.
From www.youtube.com
Breaking Surface Tension with an Infiltration Surfactant Precision How To Break Surface Tension Of Water These clusters also help reduce the surface tension of. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. As a result, objects floating in water will sink or change shape as. Due to the surface tension, small objects will float on the surface of. How To Break Surface Tension Of Water.
From ucscphysicsdemo.sites.ucsc.edu
Surface Tension of Liquids UCSC Physics Demonstration Room How To Break Surface Tension Of Water As a result, objects floating in water will sink or change shape as. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. However, touching the tent will break the surface tension, seeping water through the tent. The end. How To Break Surface Tension Of Water.
From www.animalia-life.club
Surface Tension Of Water On A Leaf How To Break Surface Tension Of Water These clusters also help reduce the surface tension of. Surfactants such as dish soap break up water’s surface tension. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. Surface tension is the energy, or work, required to increase. How To Break Surface Tension Of Water.
From ar.inspiredpencil.com
Surface Tension Of Water Diagram How To Break Surface Tension Of Water The surface tension of water bridges the pores in the finely woven material. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. As a result, objects floating in water will sink or change shape as. These clusters also help reduce the surface tension of.. How To Break Surface Tension Of Water.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How To Break Surface Tension Of Water Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. As a result, objects floating in water will sink or change shape as. Your. How To Break Surface Tension Of Water.
From www.researchgate.net
Schematic illustration of standard methods of surface tension How To Break Surface Tension Of Water Since these intermolecular forces vary. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. The surface tension of the water must be broken for sufficient gas exchange. These clusters also help reduce the surface tension of. Your goal is to reduce the surface tension of the water so that it does not support the. How To Break Surface Tension Of Water.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action How To Break Surface Tension Of Water Fortunately, creating surface agitation is easily. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Your goal is to reduce the surface tension of the water so that it does not support the formation. How To Break Surface Tension Of Water.
From sites.udel.edu
How to Teach Your Kids Science and Engineering K12 Engineering How To Break Surface Tension Of Water As a result, objects floating in water will sink or change shape as. However, touching the tent will break the surface tension, seeping water through the tent. Since these intermolecular forces vary. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. These clusters also help reduce the surface tension of. The surface tension of. How To Break Surface Tension Of Water.
From www.sciencebuddies.org
Measuring the Surface Tension of Water Science Project How To Break Surface Tension Of Water Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. However, touching the tent will break the surface tension, seeping water through the tent. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot. How To Break Surface Tension Of Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How To Break Surface Tension Of Water Surfactants such as dish soap break up water’s surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Detergent molecules' two ends make it able to break through the surface tension of water. However, touching the tent will break the surface tension, seeping water through the tent. Micelles. How To Break Surface Tension Of Water.
From www.haikudeck.com
Properties Of Water by Colten Gonzalez How To Break Surface Tension Of Water Detergent molecules' two ends make it able to break through the surface tension of water. The surface tension of the water must be broken for sufficient gas exchange. The surface tension of water bridges the pores in the finely woven material. As a result, objects floating in water will sink or change shape as. The end of the detergent molecule. How To Break Surface Tension Of Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How To Break Surface Tension Of Water Detergent molecules' two ends make it able to break through the surface tension of water. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. As a result, objects floating in water will sink or change shape as. The surface tension of water bridges the. How To Break Surface Tension Of Water.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How To Break Surface Tension Of Water However, touching the tent will break the surface tension, seeping water through the tent. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Fortunately, creating surface agitation is easily. The surface tension of the water must be broken for sufficient gas exchange. Micelles are tiny clusters of soap molecules. How To Break Surface Tension Of Water.
From www.youtube.com
DETERMINING THE SURFACE TENSION OF WATER EASY TO UNDERSTAND YouTube How To Break Surface Tension Of Water However, touching the tent will break the surface tension, seeping water through the tent. The end of the detergent molecule which attaches to fat (grease) repels. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Due to the surface tension, small objects will float on the surface of a. How To Break Surface Tension Of Water.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How To Break Surface Tension Of Water The end of the detergent molecule which attaches to fat (grease) repels. Surfactants such as dish soap break up water’s surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. However, touching the tent will break the surface tension, seeping water through the tent. Due to the surface. How To Break Surface Tension Of Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? How To Break Surface Tension Of Water Fortunately, creating surface agitation is easily. Detergent molecules' two ends make it able to break through the surface tension of water. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. As a result, objects floating in water will sink or change shape as. Since. How To Break Surface Tension Of Water.
From phys.org
Jumping water striders know how to avoid breaking of the water surface How To Break Surface Tension Of Water Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. However, touching the tent will break the surface tension, seeping water through the tent. Fortunately, creating surface agitation is easily. Detergent molecules' two ends make it able to break. How To Break Surface Tension Of Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii How To Break Surface Tension Of Water Fortunately, creating surface agitation is easily. However, touching the tent will break the surface tension, seeping water through the tent. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. The surface tension of water bridges the pores in the finely woven material. These clusters. How To Break Surface Tension Of Water.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How To Break Surface Tension Of Water Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surfactants such as dish soap break up water’s surface tension. These clusters also help reduce the surface tension of. The surface tension of the water. How To Break Surface Tension Of Water.
From www.youtube.com
What is surface tension of water explained in detail? YouTube How To Break Surface Tension Of Water Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. These clusters also help reduce the surface tension of. The end of the detergent molecule which attaches to fat (grease) repels. Since these intermolecular forces vary. Detergent molecules' two. How To Break Surface Tension Of Water.
From www.youtube.com
Slow Motion Water Droplet Falling Breaks Surface Tension and Makes How To Break Surface Tension Of Water However, touching the tent will break the surface tension, seeping water through the tent. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the. How To Break Surface Tension Of Water.
From www.youtube.com
To study surface tension of water by capillary rise method. YouTube How To Break Surface Tension Of Water These clusters also help reduce the surface tension of. However, touching the tent will break the surface tension, seeping water through the tent. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. The end of the detergent molecule which attaches to fat (grease) repels.. How To Break Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint How To Break Surface Tension Of Water These clusters also help reduce the surface tension of. As a result, objects floating in water will sink or change shape as. Since these intermolecular forces vary. Fortunately, creating surface agitation is easily. Surfactants such as dish soap break up water’s surface tension. The surface tension of the water must be broken for sufficient gas exchange. However, touching the tent. How To Break Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download How To Break Surface Tension Of Water The surface tension of water bridges the pores in the finely woven material. These clusters also help reduce the surface tension of. Due to the surface tension, small objects will float on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. Since these intermolecular forces vary. Fortunately, creating. How To Break Surface Tension Of Water.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… How To Break Surface Tension Of Water However, touching the tent will break the surface tension, seeping water through the tent. The surface tension of water bridges the pores in the finely woven material. Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. These clusters also help reduce the surface tension. How To Break Surface Tension Of Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How To Break Surface Tension Of Water Your goal is to reduce the surface tension of the water so that it does not support the formation of large bubbles or inhibit the. These clusters also help reduce the surface tension of. However, touching the tent will break the surface tension, seeping water through the tent. The surface tension of water bridges the pores in the finely woven. How To Break Surface Tension Of Water.
From mungfali.com
Water Surface Tension Bubble How To Break Surface Tension Of Water Since these intermolecular forces vary. Micelles are tiny clusters of soap molecules that help remove dirt and grime from surfaces. As a result, objects floating in water will sink or change shape as. These clusters also help reduce the surface tension of. Your goal is to reduce the surface tension of the water so that it does not support the. How To Break Surface Tension Of Water.