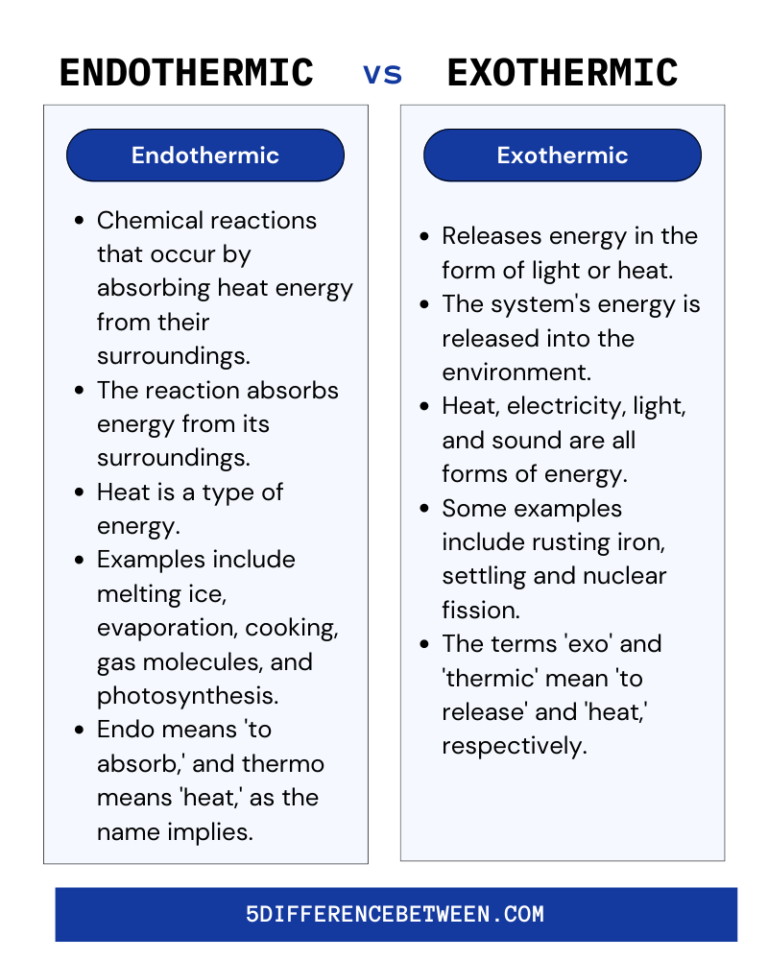
Is Burning Exothermic Or Endothermic . When a chemical reaction occurs, energy is transferred to or from the surroundings. reaction a is exothermic because heat is leaving the system making the test tube feel hot. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. Reaction b is endothermic because. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. So they’re described as exothermic reactions. all combustion reactions give off heat into their surroundings. the exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. exothermic and endothermic reactions. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and.
from 5differencebetween.com
raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. reaction a is exothermic because heat is leaving the system making the test tube feel hot. all combustion reactions give off heat into their surroundings. It releases energy by light or heat to its surrounding. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. When a chemical reaction occurs, energy is transferred to or from the surroundings. So they’re described as exothermic reactions. Reaction b is endothermic because. exothermic and endothermic reactions.
5 Difference Between Endothermic and Exothermic Reaction Endothermic
Is Burning Exothermic Or Endothermic exothermic and endothermic reactions. all combustion reactions give off heat into their surroundings. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. reaction a is exothermic because heat is leaving the system making the test tube feel hot. the exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. exothermic and endothermic reactions. Reaction b is endothermic because. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. When a chemical reaction occurs, energy is transferred to or from the surroundings. So they’re described as exothermic reactions. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Is Burning Exothermic Or Endothermic It releases energy by light or heat to its surrounding. reaction a is exothermic because heat is leaving the system making the test tube feel hot. all combustion reactions give off heat into their surroundings. Reaction b is endothermic because. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy. Is Burning Exothermic Or Endothermic.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Is Burning Exothermic Or Endothermic So they’re described as exothermic reactions. When a chemical reaction occurs, energy is transferred to or from the surroundings. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. Reaction b is endothermic because. exothermic and endothermic reactions. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions,. Is Burning Exothermic Or Endothermic.
From slideplayer.com
First Law of Thermodynamics ppt download Is Burning Exothermic Or Endothermic For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. So they’re described as exothermic reactions. exothermic and endothermic reactions. Reaction b is endothermic because. the exothermic reaction is the opposite of an endothermic reaction. reaction a is exothermic because heat is leaving the system making the test. Is Burning Exothermic Or Endothermic.
From mungfali.com
Exothermic Vs Endothermic Diagram Is Burning Exothermic Or Endothermic all combustion reactions give off heat into their surroundings. It releases energy by light or heat to its surrounding. exothermic and endothermic reactions. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. For example, when magnesium is burnt in oxygen, a very bright light is observed and a. Is Burning Exothermic Or Endothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Is Burning Exothermic Or Endothermic So they’re described as exothermic reactions. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. Reaction b is endothermic because. all combustion reactions give off heat into their surroundings. When a chemical reaction occurs, energy is transferred to or from the surroundings. the exothermic reaction is the opposite of an endothermic reaction. For. Is Burning Exothermic Or Endothermic.
From inberp.info
Differences between exothermic and endothermic reactions (2023) Is Burning Exothermic Or Endothermic all combustion reactions give off heat into their surroundings. Reaction b is endothermic because. When a chemical reaction occurs, energy is transferred to or from the surroundings. reaction a is exothermic because heat is leaving the system making the test tube feel hot. It releases energy by light or heat to its surrounding. exothermic reactions in solution. Is Burning Exothermic Or Endothermic.
From shungrill.com
Is Propane Burning In A Grill Endothermic Or Exothermic? Exploring The Is Burning Exothermic Or Endothermic the exothermic reaction is the opposite of an endothermic reaction. Reaction b is endothermic because. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. It releases energy by light or heat to its surrounding. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. . Is Burning Exothermic Or Endothermic.
From johnnycounterfit.com
Is Burning Wood Exothermic Or Endothermic Johnny Counterfit Is Burning Exothermic Or Endothermic the exothermic reaction is the opposite of an endothermic reaction. When a chemical reaction occurs, energy is transferred to or from the surroundings. It releases energy by light or heat to its surrounding. all combustion reactions give off heat into their surroundings. For example, when magnesium is burnt in oxygen, a very bright light is observed and a. Is Burning Exothermic Or Endothermic.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic Vs Exothermic Chemical Reactions Is Burning Exothermic Or Endothermic When a chemical reaction occurs, energy is transferred to or from the surroundings. Reaction b is endothermic because. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. all combustion reactions give off heat into their surroundings. So they’re described as exothermic reactions. exothermic and endothermic reactions. reaction. Is Burning Exothermic Or Endothermic.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Is Burning Exothermic Or Endothermic For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. So they’re described as exothermic reactions. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. exothermic and endothermic reactions. Reaction b is endothermic because. It releases energy by light or. Is Burning Exothermic Or Endothermic.
From www.slideserve.com
PPT Endothermic vs. Exothermic Reactions PowerPoint Presentation Is Burning Exothermic Or Endothermic exothermic and endothermic reactions. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. Reaction b is endothermic because. So they’re described as exothermic reactions. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. When a chemical reaction occurs, energy. Is Burning Exothermic Or Endothermic.
From question.pandai.org
Endothermic and exothermic reactions Is Burning Exothermic Or Endothermic So they’re described as exothermic reactions. exothermic and endothermic reactions. It releases energy by light or heat to its surrounding. the exothermic reaction is the opposite of an endothermic reaction. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. reaction a is exothermic because heat is leaving the system making the test. Is Burning Exothermic Or Endothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Is Burning Exothermic Or Endothermic the exothermic reaction is the opposite of an endothermic reaction. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. all combustion reactions give off heat into their surroundings. It releases energy by light or heat to its surrounding. raising temperature for exothermic reactions, or lowering temperature for. Is Burning Exothermic Or Endothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Is Burning Exothermic Or Endothermic For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. When a chemical reaction occurs, energy is transferred to or from the surroundings. the exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. So they’re described as exothermic reactions. . Is Burning Exothermic Or Endothermic.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Is Burning Exothermic Or Endothermic When a chemical reaction occurs, energy is transferred to or from the surroundings. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. reaction a is exothermic because heat is leaving the system making the test tube feel hot. exothermic and endothermic reactions. So they’re described as exothermic reactions. Reaction b is endothermic because.. Is Burning Exothermic Or Endothermic.
From www.diffzy.com
Exothermic vs. Endothermic Reaction What's the Difference (With Table) Is Burning Exothermic Or Endothermic reaction a is exothermic because heat is leaving the system making the test tube feel hot. It releases energy by light or heat to its surrounding. exothermic and endothermic reactions. the exothermic reaction is the opposite of an endothermic reaction. When a chemical reaction occurs, energy is transferred to or from the surroundings. So they’re described as. Is Burning Exothermic Or Endothermic.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Is Burning Exothermic Or Endothermic exothermic and endothermic reactions. Reaction b is endothermic because. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. reaction a is exothermic because heat is leaving the system making the test tube feel hot. the exothermic reaction is the opposite of an endothermic reaction. all combustion. Is Burning Exothermic Or Endothermic.
From slideplayer.com
EXOTHERMIC ENERGY TIME ppt download Is Burning Exothermic Or Endothermic exothermic and endothermic reactions. Reaction b is endothermic because. all combustion reactions give off heat into their surroundings. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. So they’re. Is Burning Exothermic Or Endothermic.
From ciplav.com
Burning A Match Endothermic Or Exothermic Is Burning Exothermic Or Endothermic So they’re described as exothermic reactions. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. exothermic and endothermic reactions. the exothermic reaction is the opposite of an endothermic reaction. It releases energy by light. Is Burning Exothermic Or Endothermic.
From www.shalom-education.com
Exothermic and Endothermic Reactions KS3 Chemistry Revision Is Burning Exothermic Or Endothermic all combustion reactions give off heat into their surroundings. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. reaction a is exothermic because heat is leaving the system making the test tube feel hot. It releases energy by light or heat to its surrounding. So they’re described as. Is Burning Exothermic Or Endothermic.
From www.slideshare.net
Thermochemistry Is Burning Exothermic Or Endothermic all combustion reactions give off heat into their surroundings. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. the exothermic reaction is the opposite of an endothermic reaction. When a chemical reaction occurs, energy is transferred to or from the surroundings. exothermic reactions in solution give out energy and the temperature increases,. Is Burning Exothermic Or Endothermic.
From www.coursehero.com
[Solved] Burning gasoline is an__________ (endothermic or exothermic Is Burning Exothermic Or Endothermic For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. Reaction b is endothermic because. exothermic and endothermic reactions. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. It releases energy by light or heat to its surrounding. all. Is Burning Exothermic Or Endothermic.
From whatsinsight.org
Endothermic vs. Exothermic with Examples What's Insight Is Burning Exothermic Or Endothermic For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. all combustion reactions give off heat into their surroundings. When a chemical reaction occurs, energy is transferred to or from the surroundings. reaction a is exothermic because heat is leaving the system making the test tube feel hot. It. Is Burning Exothermic Or Endothermic.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Is Burning Exothermic Or Endothermic It releases energy by light or heat to its surrounding. exothermic and endothermic reactions. So they’re described as exothermic reactions. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. all combustion reactions give off heat into their surroundings. reaction a is exothermic because heat is leaving the. Is Burning Exothermic Or Endothermic.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Is Burning Exothermic Or Endothermic So they’re described as exothermic reactions. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. When a chemical reaction occurs, energy is transferred to or from the surroundings. reaction a is exothermic because heat is leaving the system making the test tube feel hot. It releases energy by light or heat to its surrounding.. Is Burning Exothermic Or Endothermic.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Is Burning Exothermic Or Endothermic When a chemical reaction occurs, energy is transferred to or from the surroundings. So they’re described as exothermic reactions. It releases energy by light or heat to its surrounding. Reaction b is endothermic because. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. reaction a is exothermic because heat is leaving the system making. Is Burning Exothermic Or Endothermic.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Is Burning Exothermic Or Endothermic exothermic and endothermic reactions. It releases energy by light or heat to its surrounding. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. reaction a is exothermic because heat is leaving the system making the test tube feel hot. Reaction b is endothermic because. When a chemical reaction. Is Burning Exothermic Or Endothermic.
From slideplayer.com
Energy, Temperature, And Heat. ppt download Is Burning Exothermic Or Endothermic raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. When a chemical reaction occurs, energy is transferred to or from the surroundings. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. exothermic and endothermic reactions. reaction a is exothermic because heat is leaving. Is Burning Exothermic Or Endothermic.
From www.worksheetsplanet.com
What is an Exothermic Reaction Definition and Example Is Burning Exothermic Or Endothermic reaction a is exothermic because heat is leaving the system making the test tube feel hot. So they’re described as exothermic reactions. all combustion reactions give off heat into their surroundings. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. Reaction b is endothermic because. exothermic and. Is Burning Exothermic Or Endothermic.
From slideplayer.com
CHAPTER ppt download Is Burning Exothermic Or Endothermic raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. It releases energy by light or heat to its surrounding. When a chemical reaction occurs, energy is transferred to or from the surroundings. Reaction b is endothermic because. So they’re described as exothermic reactions. reaction a is exothermic because heat is leaving the system making. Is Burning Exothermic Or Endothermic.
From www.slideserve.com
PPT ENTHALPY OF FORMATION Combustion of Methanol PowerPoint Is Burning Exothermic Or Endothermic the exothermic reaction is the opposite of an endothermic reaction. all combustion reactions give off heat into their surroundings. reaction a is exothermic because heat is leaving the system making the test tube feel hot. exothermic and endothermic reactions. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Is Burning Exothermic Or Endothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Is Burning Exothermic Or Endothermic all combustion reactions give off heat into their surroundings. exothermic and endothermic reactions. reaction a is exothermic because heat is leaving the system making the test tube feel hot. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of. So they’re described as exothermic reactions. When a chemical. Is Burning Exothermic Or Endothermic.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Is Burning Exothermic Or Endothermic raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. reaction a is exothermic because heat is leaving the system making the test tube feel hot. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. It releases energy by light or heat to its surrounding.. Is Burning Exothermic Or Endothermic.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Is Burning Exothermic Or Endothermic When a chemical reaction occurs, energy is transferred to or from the surroundings. exothermic and endothermic reactions. raising temperature for exothermic reactions, or lowering temperature for endothermic reactions, causes the. It releases energy by light or heat to its surrounding. the exothermic reaction is the opposite of an endothermic reaction. For example, when magnesium is burnt in. Is Burning Exothermic Or Endothermic.
From www.slideserve.com
PPT 7.3 Exothermic and Endothermic Reactions PowerPoint Presentation Is Burning Exothermic Or Endothermic It releases energy by light or heat to its surrounding. exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and. all combustion reactions give off heat into their surroundings. So they’re described as exothermic reactions. exothermic and endothermic reactions. raising temperature for exothermic reactions, or lowering temperature for. Is Burning Exothermic Or Endothermic.