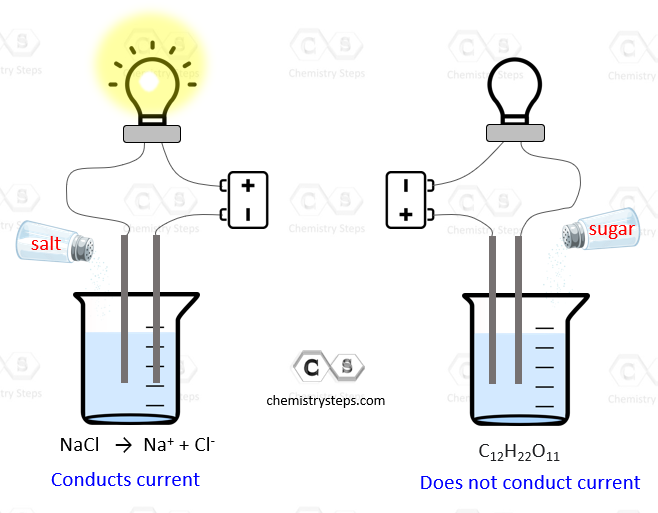
Example Of Strong Electrolyte In Our Daily Life . Electrolytes are examples of minerals. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. Aqueous solutions containing electrolytes conduct electricity. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. It’s because every cell in your body uses them! From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. The solution will contain only ions and no molecules of the. Electrolytes are chemicals that break into ions in water. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Examples of strong electrolytes include strong acids, strong bases, and salts. When these substances dissolve in water,.
from general.chemistrysteps.com
Electrolytes are examples of minerals. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Aqueous solutions containing electrolytes conduct electricity. The solution will contain only ions and no molecules of the. Examples of strong electrolytes include strong acids, strong bases, and salts. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. When these substances dissolve in water,. Electrolytes are chemicals that break into ions in water.
Strong and Weak Electrolytes Chemistry Steps
Example Of Strong Electrolyte In Our Daily Life The solution will contain only ions and no molecules of the. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. Aqueous solutions containing electrolytes conduct electricity. When these substances dissolve in water,. Examples of strong electrolytes include strong acids, strong bases, and salts. It’s because every cell in your body uses them! The solution will contain only ions and no molecules of the. Electrolytes are examples of minerals. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. Electrolytes are chemicals that break into ions in water. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution.
From healthjade.com
Electrolytes Sources and Foods High In Electrolytes Example Of Strong Electrolyte In Our Daily Life From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. The solution will contain only ions and no. Example Of Strong Electrolyte In Our Daily Life.
From ecurrencythailand.com
Which Is The Strongest Electrolyte? 10 Most Correct Answers Example Of Strong Electrolyte In Our Daily Life An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Examples of strong electrolytes include strong acids, strong bases, and salts. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. Electrolytes are examples of minerals. From simple tasks like typing at a keyboard to. Example Of Strong Electrolyte In Our Daily Life.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Example Of Strong Electrolyte In Our Daily Life Aqueous solutions containing electrolytes conduct electricity. Electrolytes are examples of minerals. Electrolytes are chemicals that break into ions in water. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. It’s because every cell in. Example Of Strong Electrolyte In Our Daily Life.
From lessonschoolcomminuted.z13.web.core.windows.net
Fluid And Electrolyte Quizzes Example Of Strong Electrolyte In Our Daily Life From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. Examples of strong electrolytes include strong acids, strong bases, and salts. It’s because every cell in your body uses them! Electrolytes are chemicals that break. Example Of Strong Electrolyte In Our Daily Life.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation ID159152 Example Of Strong Electrolyte In Our Daily Life When these substances dissolve in water,. Examples of strong electrolytes include strong acids, strong bases, and salts. It’s because every cell in your body uses them! A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Aqueous solutions containing electrolytes conduct electricity. An ionic compound for example, sodium chloride dissolved in water is. Example Of Strong Electrolyte In Our Daily Life.
From gamesmartz.com
Strong Electrolyte Definition & Image GameSmartz Example Of Strong Electrolyte In Our Daily Life Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. It’s because every cell in your body uses them! Electrolytes are chemicals that break into ions in water. The solution will contain only ions and no molecules of the. Examples of strong electrolytes include strong acids, strong bases, and salts. A strong electrolyte is a. Example Of Strong Electrolyte In Our Daily Life.
From circuitdburticant.z13.web.core.windows.net
Strong Electrolyte Diagram Example Of Strong Electrolyte In Our Daily Life Electrolytes are chemicals that break into ions in water. Electrolytes are examples of minerals. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. It’s because every cell in your body uses them! Aqueous solutions containing electrolytes conduct electricity. When these substances dissolve in water,. From simple tasks like typing at a. Example Of Strong Electrolyte In Our Daily Life.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Example Of Strong Electrolyte In Our Daily Life A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. When these substances dissolve in water,. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. It’s because every cell in your body uses them! From simple tasks like typing at a keyboard to complex. Example Of Strong Electrolyte In Our Daily Life.
From general.chemistrysteps.com
Strong and Weak Electrolytes Chemistry Steps Example Of Strong Electrolyte In Our Daily Life Electrolytes are chemicals that break into ions in water. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. Examples. Example Of Strong Electrolyte In Our Daily Life.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Example Of Strong Electrolyte In Our Daily Life Electrolytes are examples of minerals. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity.. Example Of Strong Electrolyte In Our Daily Life.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Example Of Strong Electrolyte In Our Daily Life A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. The solution will contain only ions and no molecules of the. Aqueous solutions containing electrolytes conduct electricity. Electrolytes are examples of minerals. It’s because every cell in your body uses them! Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles,. Example Of Strong Electrolyte In Our Daily Life.
From www.osmosis.org
Overview of Electrolyte Balance Osmosis Video Library Example Of Strong Electrolyte In Our Daily Life The solution will contain only ions and no molecules of the. When these substances dissolve in water,. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. Electrolytes are chemicals that break into ions in water. Aqueous solutions containing electrolytes conduct electricity. Examples of strong electrolytes include strong acids, strong bases, and salts. An ionic. Example Of Strong Electrolyte In Our Daily Life.
From in.pinterest.com
Know Why Electrolytes Are Important For You To Stay Healthy Example Of Strong Electrolyte In Our Daily Life Examples of strong electrolytes include strong acids, strong bases, and salts. When these substances dissolve in water,. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. The solution will contain only ions and no molecules of the. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and. Example Of Strong Electrolyte In Our Daily Life.
From www.ausmed.co.nz
Electrolyte Imbalances, Ranges & Disturbances Ausmed Example Of Strong Electrolyte In Our Daily Life It’s because every cell in your body uses them! Examples of strong electrolytes include strong acids, strong bases, and salts. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Aqueous solutions containing electrolytes conduct electricity. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. Electrolytes. Example Of Strong Electrolyte In Our Daily Life.
From www.numerade.com
SOLVED4) Which compounds are strong (electrolyte) acids and weak Example Of Strong Electrolyte In Our Daily Life When these substances dissolve in water,. Electrolytes are examples of minerals. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. From simple tasks like typing at a keyboard to complex movements like burpees, and. Example Of Strong Electrolyte In Our Daily Life.
From www.slideserve.com
PPT Weak, Strong and Nonelectrolytes PowerPoint Presentation, free Example Of Strong Electrolyte In Our Daily Life Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. Electrolytes are examples of minerals. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. The solution will. Example Of Strong Electrolyte In Our Daily Life.
From www.phartoonz.com
List of electrolytes and their normal range Phartoonz Example Of Strong Electrolyte In Our Daily Life Examples of strong electrolytes include strong acids, strong bases, and salts. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. It’s because every cell in your body uses them! When these substances dissolve in water,. A strong. Example Of Strong Electrolyte In Our Daily Life.
From gamesmartz.com
Weak Electrolyte Definition & Image GameSmartz Example Of Strong Electrolyte In Our Daily Life Electrolytes are examples of minerals. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. The solution will contain only ions and no molecules of the. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. A strong electrolyte is a solute. Example Of Strong Electrolyte In Our Daily Life.
From www.youtube.com
Uses of Electrochemistry in our daily life (Electrochemistry part 2 for Example Of Strong Electrolyte In Our Daily Life It’s because every cell in your body uses them! When these substances dissolve in water,. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. The solution will contain only ions and no molecules of the. Examples of strong electrolytes include strong acids, strong bases, and salts. Electrolytes are crucial for body processes. Example Of Strong Electrolyte In Our Daily Life.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Example Of Strong Electrolyte In Our Daily Life Examples of strong electrolytes include strong acids, strong bases, and salts. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. Electrolytes are chemicals that break into ions in water. Aqueous solutions containing electrolytes conduct electricity. It’s because every cell in your body uses them! Electrolytes are crucial for body processes like conducting. Example Of Strong Electrolyte In Our Daily Life.
From www.shutterstock.com
19 Strong Electrolyte And Weak Electrolyte Images, Stock Photos Example Of Strong Electrolyte In Our Daily Life When these substances dissolve in water,. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Examples of strong electrolytes include strong acids, strong bases, and salts. From simple tasks like typing at a keyboard to complex movements. Example Of Strong Electrolyte In Our Daily Life.
From www.vrogue.co
Weak Electrolyte Definition And Examples vrogue.co Example Of Strong Electrolyte In Our Daily Life From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. Aqueous solutions containing electrolytes conduct electricity. When these substances dissolve in water,. Examples of strong electrolytes include strong acids, strong bases, and salts. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution.. Example Of Strong Electrolyte In Our Daily Life.
From www.numerade.com
SOLVEDWhat is an electrolyte? How can we differentiate between a Example Of Strong Electrolyte In Our Daily Life It’s because every cell in your body uses them! Aqueous solutions containing electrolytes conduct electricity. When these substances dissolve in water,. Electrolytes are chemicals that break into ions in water. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. They are distinguished from other minerals in that they carry an electrical charge. Example Of Strong Electrolyte In Our Daily Life.
From melissa-media.blogspot.com
Melissa Media Strong And Weak Electrolytes Example Of Strong Electrolyte In Our Daily Life Aqueous solutions containing electrolytes conduct electricity. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. Electrolytes are examples of minerals. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water.. Example Of Strong Electrolyte In Our Daily Life.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Example Of Strong Electrolyte In Our Daily Life A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Electrolytes are chemicals that break. Example Of Strong Electrolyte In Our Daily Life.
From www.vrogue.co
A Compound Of Low Solubility A Is Always A Strong Ele vrogue.co Example Of Strong Electrolyte In Our Daily Life Aqueous solutions containing electrolytes conduct electricity. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Electrolytes are examples of minerals. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. The solution will contain only ions and no molecules of the. From simple tasks like. Example Of Strong Electrolyte In Our Daily Life.
From www.vrogue.co
Strong Electrolyte Definition And Examples vrogue.co Example Of Strong Electrolyte In Our Daily Life From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. A strong electrolyte is a. Example Of Strong Electrolyte In Our Daily Life.
From www.vrogue.co
Strong Electrolyte Definition And Examples vrogue.co Example Of Strong Electrolyte In Our Daily Life Aqueous solutions containing electrolytes conduct electricity. Electrolytes are chemicals that break into ions in water. When these substances dissolve in water,. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. An ionic. Example Of Strong Electrolyte In Our Daily Life.
From www.teachoo.com
10+ Acids used in Daily Life (with images) Teachoo Science Example Of Strong Electrolyte In Our Daily Life Electrolytes are chemicals that break into ions in water. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Electrolytes are examples of minerals. Examples of strong electrolytes include strong acids, strong. Example Of Strong Electrolyte In Our Daily Life.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Example Of Strong Electrolyte In Our Daily Life The solution will contain only ions and no molecules of the. It’s because every cell in your body uses them! Aqueous solutions containing electrolytes conduct electricity. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Electrolytes are chemicals that break into ions in water. Electrolytes are crucial for body processes like conducting. Example Of Strong Electrolyte In Our Daily Life.
From www.vrogue.co
Weak Electrolyte Definition And Examples vrogue.co Example Of Strong Electrolyte In Our Daily Life A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. Electrolytes are examples of minerals. The solution will contain only ions and no molecules of the. It’s because every cell in your body uses them! Electrolytes are chemicals that break into ions in water. Electrolytes are crucial for body processes like conducting nerve. Example Of Strong Electrolyte In Our Daily Life.
From www.toppr.com
Name the three types of electrolytes. Give an example each. Example Of Strong Electrolyte In Our Daily Life The solution will contain only ions and no molecules of the. When these substances dissolve in water,. Aqueous solutions containing electrolytes conduct electricity. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. Electrolytes are crucial for body processes like conducting nerve impulses, contracting muscles, hydrating, and regulating. It’s because every cell in. Example Of Strong Electrolyte In Our Daily Life.
From in.pinterest.com
Difference Between Strong and Weak Electrolytes Definition Example Of Strong Electrolyte In Our Daily Life From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. The solution will contain only ions and no molecules of the. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Electrolytes are examples of minerals. Electrolytes are chemicals that break into. Example Of Strong Electrolyte In Our Daily Life.
From www.youtube.com
Strong Electrolytes YouTube Example Of Strong Electrolyte In Our Daily Life It’s because every cell in your body uses them! Electrolytes are examples of minerals. From simple tasks like typing at a keyboard to complex movements like burpees, and every vital process in between, electrolyte. Electrolytes are chemicals that break into ions in water. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution.. Example Of Strong Electrolyte In Our Daily Life.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Example Of Strong Electrolyte In Our Daily Life A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in solution. When these substances dissolve in water,. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. Electrolytes are examples of minerals. Electrolytes are chemicals that break into ions in water. The solution will contain only ions. Example Of Strong Electrolyte In Our Daily Life.