What Is Electrochemical Cell With Example . Describe what you know about an electrochemical cell. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. This kind of cell includes the galvanic, or voltaic, cell, named after. What processes are responsible for conduction of electricity in an electrochemical cell? What is an electrochemical cell? If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate.
from www.youtube.com
What processes are responsible for conduction of electricity in an electrochemical cell? An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. What is an electrochemical cell? Describe what you know about an electrochemical cell. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate.
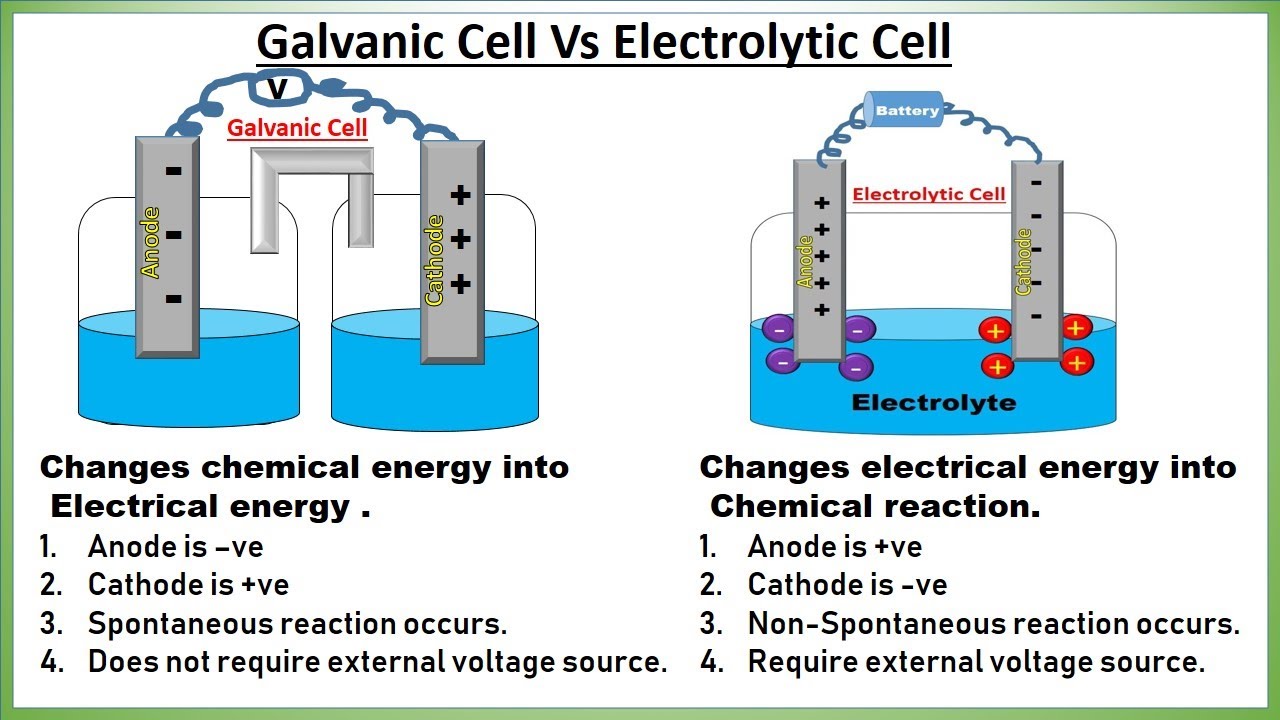
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells
What Is Electrochemical Cell With Example This kind of cell includes the galvanic, or voltaic, cell, named after. Describe what you know about an electrochemical cell. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. What is an electrochemical cell? If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. This kind of cell includes the galvanic, or voltaic, cell, named after. What processes are responsible for conduction of electricity in an electrochemical cell?
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is Electrochemical Cell With Example An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What processes are responsible for conduction of electricity in an electrochemical cell? If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. This kind of cell includes. What Is Electrochemical Cell With Example.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. This kind of cell includes the galvanic, or voltaic, cell, named after. What is an electrochemical cell? An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. Describe what you know about an. What Is Electrochemical Cell With Example.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. What is an electrochemical cell? If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Describe what you know about an electrochemical cell. An electrochemical. What Is Electrochemical Cell With Example.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is Electrochemical Cell With Example Describe what you know about an electrochemical cell. What processes are responsible for conduction of electricity in an electrochemical cell? An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. What is an electrochemical cell? An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. If a. What Is Electrochemical Cell With Example.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses What Is Electrochemical Cell With Example What is an electrochemical cell? Describe what you know about an electrochemical cell. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. An electrochemical. What Is Electrochemical Cell With Example.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 What Is Electrochemical Cell With Example Describe what you know about an electrochemical cell. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. This kind of cell includes the galvanic, or voltaic, cell, named after. What processes are responsible for conduction of electricity in an electrochemical cell? An electrochemical cell is. What Is Electrochemical Cell With Example.
From mavink.com
Electrochemical Cell Diagram What Is Electrochemical Cell With Example What is an electrochemical cell? An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. Describe what you know about an electrochemical cell. What processes are responsible for conduction of electricity in an electrochemical cell? An electrochemical. What Is Electrochemical Cell With Example.
From www.chemicals.co.uk
Measuring The EMF Of An Electrochemical Cell The Chemistry Blog What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. What is an electrochemical cell? What processes are responsible for conduction of electricity in an electrochemical cell? An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. Describe what you know about. What Is Electrochemical Cell With Example.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector What Is Electrochemical Cell With Example If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Describe what you know about an electrochemical cell. What is an electrochemical cell? An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. An extremely important class of oxidation and. What Is Electrochemical Cell With Example.
From classfullfauvette.z13.web.core.windows.net
Examples Of Electrochemical Cell What Is Electrochemical Cell With Example An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. This kind of. What Is Electrochemical Cell With Example.
From www.slideshare.net
Electrochemistry electrochemical cells What Is Electrochemical Cell With Example An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. What is an electrochemical cell? What processes are responsible for conduction of electricity in an electrochemical cell? Describe what you know about an electrochemical cell. If a redox reaction can be split into half reactions it becomes possible to build a device, called. What Is Electrochemical Cell With Example.
From saylordotorg.github.io
Electrochemistry What Is Electrochemical Cell With Example This kind of cell includes the galvanic, or voltaic, cell, named after. Describe what you know about an electrochemical cell. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. What processes are responsible for conduction of. What Is Electrochemical Cell With Example.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is Electrochemical Cell With Example Describe what you know about an electrochemical cell. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. What is an electrochemical cell? An extremely important class of oxidation and reduction reactions are used to provide useful. What Is Electrochemical Cell With Example.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells What Is Electrochemical Cell With Example If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to. What Is Electrochemical Cell With Example.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples What Is Electrochemical Cell With Example An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. This kind of cell includes the galvanic, or voltaic, cell, named after. What is an electrochemical cell? An electrochemical cell is a device. What Is Electrochemical Cell With Example.
From studylib.net
Electrochemical cells What Is Electrochemical Cell With Example Describe what you know about an electrochemical cell. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. What processes are responsible for conduction of electricity in an electrochemical cell? What is an electrochemical cell? An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox. What Is Electrochemical Cell With Example.
From www.youtube.com
What is Electrochemical Cell Notation Line notation Cell Diagram What Is Electrochemical Cell With Example An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe what you know about an electrochemical cell.. What Is Electrochemical Cell With Example.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What processes are. What Is Electrochemical Cell With Example.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrochemical Cell With Example What processes are responsible for conduction of electricity in an electrochemical cell? Describe what you know about an electrochemical cell. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. An extremely important class of oxidation and reduction reactions. What Is Electrochemical Cell With Example.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. What processes are responsible for conduction of electricity in an electrochemical cell? An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What is an electrochemical cell? An electrochemical cell. What Is Electrochemical Cell With Example.
From www.youtube.com
Electrochemistry 04 Writing Electrochemical Cell Notation YouTube What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Describe what you know about an electrochemical cell. This kind of cell includes the galvanic, or voltaic, cell, named after. If a redox reaction can be split into half reactions it becomes possible to build a device, called an. What Is Electrochemical Cell With Example.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. What is an electrochemical cell? An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. What processes are responsible for conduction of electricity in an electrochemical cell? An extremely important class of oxidation. What Is Electrochemical Cell With Example.
From www.pearson.com
Electrochemical Cells Video Tutorial & Practice Channels for Pearson+ What Is Electrochemical Cell With Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has. What Is Electrochemical Cell With Example.
From www.pinterest.com
Electrochemical Cells Chemwiki Chemistry Textbook, Gcse Chemistry What Is Electrochemical Cell With Example If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. What processes are responsible for conduction of electricity in an electrochemical cell? Describe what you know about an electrochemical cell. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy. What Is Electrochemical Cell With Example.
From mavink.com
Diagram Of Electrochemical Cell What Is Electrochemical Cell With Example What processes are responsible for conduction of electricity in an electrochemical cell? If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device which. What Is Electrochemical Cell With Example.
From mmerevise.co.uk
Electrochemical Cells MME What Is Electrochemical Cell With Example An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. What processes are responsible for conduction of electricity in an electrochemical cell? An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device which either uses chemical. What Is Electrochemical Cell With Example.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material What Is Electrochemical Cell With Example An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. If a redox reaction can be split into. What Is Electrochemical Cell With Example.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID What Is Electrochemical Cell With Example What processes are responsible for conduction of electricity in an electrochemical cell? If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An extremely important. What Is Electrochemical Cell With Example.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at What Is Electrochemical Cell With Example This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. What is an electrochemical cell? What processes are responsible for conduction of electricity in an electrochemical cell? If a redox reaction can be split into half reactions it becomes possible to build. What Is Electrochemical Cell With Example.
From classfullbooklice.z13.web.core.windows.net
Working Of Electrochemical Cell What Is Electrochemical Cell With Example What is an electrochemical cell? This kind of cell includes the galvanic, or voltaic, cell, named after. What processes are responsible for conduction of electricity in an electrochemical cell? An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An apparatus that is used to generate electricity from a spontaneous redox reaction or,. What Is Electrochemical Cell With Example.
From chemwiki.ucdavis.edu
Voltaic Cells Chemwiki What Is Electrochemical Cell With Example What processes are responsible for conduction of electricity in an electrochemical cell? An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. This kind of cell includes the galvanic, or voltaic, cell, named after. If a redox. What Is Electrochemical Cell With Example.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General What Is Electrochemical Cell With Example An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. What is an electrochemical cell? An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox. What Is Electrochemical Cell With Example.
From guidelibauditioned.z13.web.core.windows.net
Electrochemical Cell Diagram What Is Electrochemical Cell With Example If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. What is an electrochemical cell? An electrochemical cell is a device which either uses chemical reactions occurring in it to generate. Describe what you know about an electrochemical cell. This kind of cell includes the galvanic,. What Is Electrochemical Cell With Example.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson What Is Electrochemical Cell With Example What processes are responsible for conduction of electricity in an electrochemical cell? What is an electrochemical cell? An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Describe what you know about an electrochemical cell. This kind of cell includes the galvanic, or voltaic, cell, named after. An extremely. What Is Electrochemical Cell With Example.
From www.alamy.com
Basic components of an electrochemical cell Stock Photo Alamy What Is Electrochemical Cell With Example Describe what you know about an electrochemical cell. What processes are responsible for conduction of electricity in an electrochemical cell? What is an electrochemical cell? This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An extremely important class. What Is Electrochemical Cell With Example.