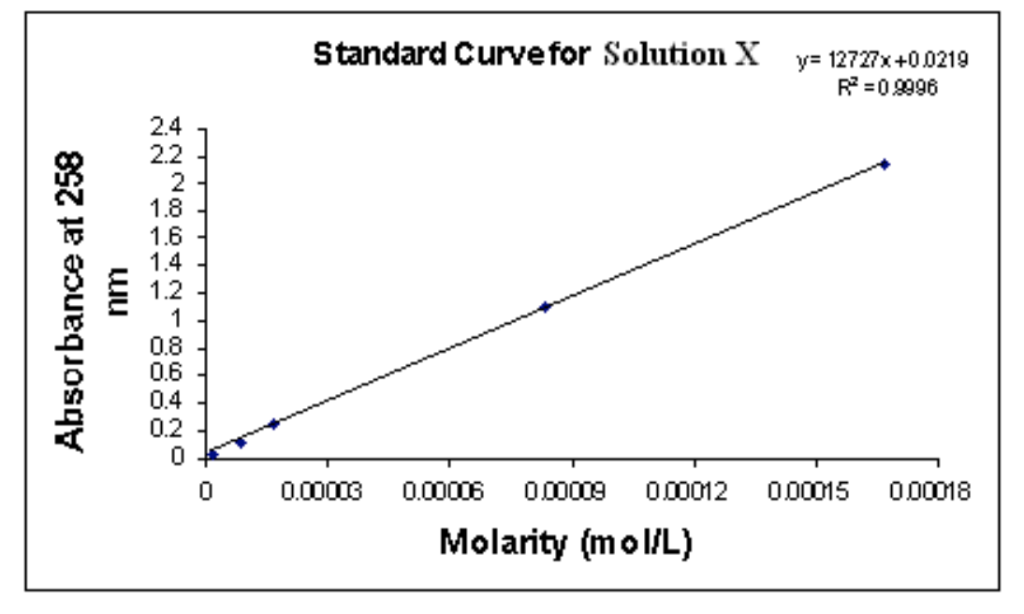
Standard Curve And Spectrophotometry . Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. As you continue your research, you measure the bacterial samples using the spectrophotometer. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. It calculates the concentration of the unknown. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Every standard curve is generated. Use the spectrophotometer to measure the absorbance of a solution. Use the standard curve you created to determine the amount of cfus.
from www.chegg.com
Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. Use the spectrophotometer to measure the absorbance of a solution. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. As you continue your research, you measure the bacterial samples using the spectrophotometer. It calculates the concentration of the unknown. Use the standard curve you created to determine the amount of cfus. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Every standard curve is generated.
Solved a.) what is the purpose of a standard curve for an
Standard Curve And Spectrophotometry It calculates the concentration of the unknown. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Every standard curve is generated. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. It calculates the concentration of the unknown. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Use the spectrophotometer to measure the absorbance of a solution. Use the standard curve you created to determine the amount of cfus. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. As you continue your research, you measure the bacterial samples using the spectrophotometer.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID506114 Standard Curve And Spectrophotometry Use the standard curve you created to determine the amount of cfus. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Use the spectrophotometer to measure the absorbance of a solution. Use excel and make a standard curve and use the r2 value to evaluate the. Standard Curve And Spectrophotometry.
From www.studocu.com
Lab manual 2 spectrophotometry class lectures and summaries Standard Curve And Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use the standard curve you created to determine the amount of cfus. Spectrophotometric analysis is essential for. Standard Curve And Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID506114 Standard Curve And Spectrophotometry As you continue your research, you measure the bacterial samples using the spectrophotometer. Use the spectrophotometer to measure the absorbance of a solution. Use the standard curve you created to determine the amount of cfus. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. In this exercise you will. Standard Curve And Spectrophotometry.
From www.youtube.com
Lab Review Standard Curve (Unit 2 Spectrophotometry) YouTube Standard Curve And Spectrophotometry Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. Use the spectrophotometer to measure the absorbance of a solution. Every standard curve is generated. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Spectrophotometric analysis is. Standard Curve And Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID506114 Standard Curve And Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Use the standard curve you created to determine the amount of cfus. Every standard curve is generated. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance.. Standard Curve And Spectrophotometry.
From www.researchgate.net
Standard curve of ethanol. Download Scientific Diagram Standard Curve And Spectrophotometry Use the standard curve you created to determine the amount of cfus. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. In this exercise you will determine. Standard Curve And Spectrophotometry.
From www.chegg.com
Solved Spectrophotometry HomeworkA) Plot the Standard Curve Standard Curve And Spectrophotometry Every standard curve is generated. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Use the standard curve you created to determine the amount of cfus.. Standard Curve And Spectrophotometry.
From www.chegg.com
Solved Spectrophotometry Lab Assignment.Maltose standard Standard Curve And Spectrophotometry Use the standard curve you created to determine the amount of cfus. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Every standard curve is generated. It calculates the concentration of the unknown. Standard curves are graphs of light absorbance versus solution concentration which can be. Standard Curve And Spectrophotometry.
From www.chegg.com
Solved a.) what is the purpose of a standard curve for an Standard Curve And Spectrophotometry Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Every standard curve is generated. Standard curves are graphs of light absorbance versus solution concentration which can be. Standard Curve And Spectrophotometry.
From www.researchgate.net
Standard calibration Curve of Levodopa using UVVisible... Download Standard Curve And Spectrophotometry In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use the spectrophotometer to measure the absorbance of a solution. Standard curves are graphs of light absorbance. Standard Curve And Spectrophotometry.
From gamma.app
Spectrophotometry and Calibration Curves Standard Curve And Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Use the standard curve you created to determine the amount of cfus. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. Every standard curve is generated. Spectrophotometric. Standard Curve And Spectrophotometry.
From www.scribd.com
Quantifying Glucose Concentration Through Spectrophotometry A Standard Standard Curve And Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Use the spectrophotometer to measure the absorbance of a solution. Use the standard curve you created to determine. Standard Curve And Spectrophotometry.
From www.researchgate.net
A.1) UV. Spectrophotometer Calibration Curve Download Scientific Diagram Standard Curve And Spectrophotometry Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Every standard curve is generated. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use the standard curve you created to determine the amount of cfus. Standard curves are graphs. Standard Curve And Spectrophotometry.
From www.slideserve.com
PPT SPECTROPHOTOMETRY IN BIOTECHNOLOGY PowerPoint Presentation, free Standard Curve And Spectrophotometry A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Every standard curve is generated. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a. Standard Curve And Spectrophotometry.
From www.researchgate.net
Standard curve obtained from known concentrations of DOX analyzed by Standard Curve And Spectrophotometry Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Use the standard curve you created to determine the amount of cfus. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. As you continue your research, you measure the bacterial. Standard Curve And Spectrophotometry.
From www.researchgate.net
Standard calibration curve for ampicillin by UV absorption Standard Curve And Spectrophotometry As you continue your research, you measure the bacterial samples using the spectrophotometer. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. Use the standard curve you. Standard Curve And Spectrophotometry.
From www.mdpi.com
Molecules Free FullText Spectrophotometric Determination of Standard Curve And Spectrophotometry In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Use the spectrophotometer to measure the absorbance of a solution. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. Use the standard curve you created to determine. Standard Curve And Spectrophotometry.
From www.researchgate.net
Uvvis spectrophotometry curves of PNP (a) scanning curve and (b Standard Curve And Spectrophotometry In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. A calibration curve, also known as a standard curve, is a way to identify. Standard Curve And Spectrophotometry.
From www.scielo.br
SciELO Brasil Use of different matrices to construct the standard Standard Curve And Spectrophotometry Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. As you continue your research, you measure the bacterial samples using the spectrophotometer. Use excel and make a standard curve and. Standard Curve And Spectrophotometry.
From www.techwalla.com
How to Plot a Standard Curve in Excel Standard Curve And Spectrophotometry A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use the standard curve you created to determine the amount of cfus. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. In this exercise you will determine the unique “fingerprint”. Standard Curve And Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID506114 Standard Curve And Spectrophotometry It calculates the concentration of the unknown. As you continue your research, you measure the bacterial samples using the spectrophotometer. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Use the standard curve you created to determine the amount of cfus. Use the spectrophotometer to measure. Standard Curve And Spectrophotometry.
From www.researchgate.net
KSLW standard curves (280 nm). (a) highperformance liquid Standard Curve And Spectrophotometry As you continue your research, you measure the bacterial samples using the spectrophotometer. It calculates the concentration of the unknown. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in. Standard Curve And Spectrophotometry.
From www.vrogue.co
Uv Vis Spectrophotometry Curves Of Pnp A Scanning Cur vrogue.co Standard Curve And Spectrophotometry Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Use the spectrophotometer to measure the absorbance of a solution. Every standard curve is generated. Use excel and. Standard Curve And Spectrophotometry.
From chem.libretexts.org
4.4 UVVisible Spectroscopy Chemistry LibreTexts Standard Curve And Spectrophotometry In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Every standard curve is generated. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and. Standard Curve And Spectrophotometry.
From www.youtube.com
BI308 skills for Bioscientists spectrophotometry and standard Standard Curve And Spectrophotometry As you continue your research, you measure the bacterial samples using the spectrophotometer. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Every standard curve is generated. Use the standard curve you created to determine the amount of cfus. Use excel and make a standard curve. Standard Curve And Spectrophotometry.
From ambitiousmares.blogspot.com
32 Label The Graph Below With The Correct Axes For A Standard Curve Standard Curve And Spectrophotometry Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Use the standard curve you created to determine the amount of cfus. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. In this exercise you will determine. Standard Curve And Spectrophotometry.
From ambitiousmares.blogspot.com
32 Label The Graph Below With The Correct Axes For A Standard Curve Standard Curve And Spectrophotometry Every standard curve is generated. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. In this exercise you will determine the unique “fingerprint” for a colored molecule. Standard Curve And Spectrophotometry.
From www.researchgate.net
Establishment of the standard curves according to the HPLC and UVVis Standard Curve And Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. As you continue your research, you measure the bacterial samples using the spectrophotometer. Use the standard curve you created to determine the amount of cfus. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and. Standard Curve And Spectrophotometry.
From www.scielo.br
SciELO Brasil Use of different matrices to construct the standard Standard Curve And Spectrophotometry It calculates the concentration of the unknown. As you continue your research, you measure the bacterial samples using the spectrophotometer. Every standard curve is generated. Use the spectrophotometer to measure the absorbance of a solution. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. In this exercise you will determine. Standard Curve And Spectrophotometry.
From www.researchgate.net
Calibration graph for the spectrophotometric determination of AMX Standard Curve And Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Use the standard curve you created to determine the amount of cfus. Spectrophotometric analysis. Standard Curve And Spectrophotometry.
From www.researchgate.net
Fluorescent spectra (a) and linear calibration plot (b) of the Standard Curve And Spectrophotometry Every standard curve is generated. Use the spectrophotometer to measure the absorbance of a solution. As you continue your research, you measure the bacterial samples using the spectrophotometer. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use the standard curve you created to determine the amount of cfus.. Standard Curve And Spectrophotometry.
From www.researchgate.net
Standard curve of standard C. oleifera saponins by UV spectrophotometry Standard Curve And Spectrophotometry Use the standard curve you created to determine the amount of cfus. Every standard curve is generated. As you continue your research, you measure the bacterial samples using the spectrophotometer. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Use the spectrophotometer to measure the absorbance of a solution. In. Standard Curve And Spectrophotometry.
From www.slideserve.com
PPT SPECTROSCOPY 101 PowerPoint Presentation, free download ID2427178 Standard Curve And Spectrophotometry Every standard curve is generated. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. As you continue your research, you measure the bacterial samples using the spectrophotometer. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular. Use the spectrophotometer to. Standard Curve And Spectrophotometry.
From www.scribd.com
Standard Curve Lab 1 PDF Spectrophotometry Analysis Standard Curve And Spectrophotometry Every standard curve is generated. As you continue your research, you measure the bacterial samples using the spectrophotometer. In this exercise you will determine the unique “fingerprint” for a colored molecule and use a spectrophotometer to measure the concentration of a. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and molecular.. Standard Curve And Spectrophotometry.
From www.studocu.com
Pre ii Standard Curve new Experiment (ii) 2019 S Avzianova Standard Curve And Spectrophotometry Standard curves are graphs of light absorbance versus solution concentration which can be used to figure out the solute concentration in unknown samples. Use excel and make a standard curve and use the r2 value to evaluate the quality of the standard. Spectrophotometric analysis is essential for determining biomolecule concentration of a solution and is employed ubiquitously in biochemistry and. Standard Curve And Spectrophotometry.