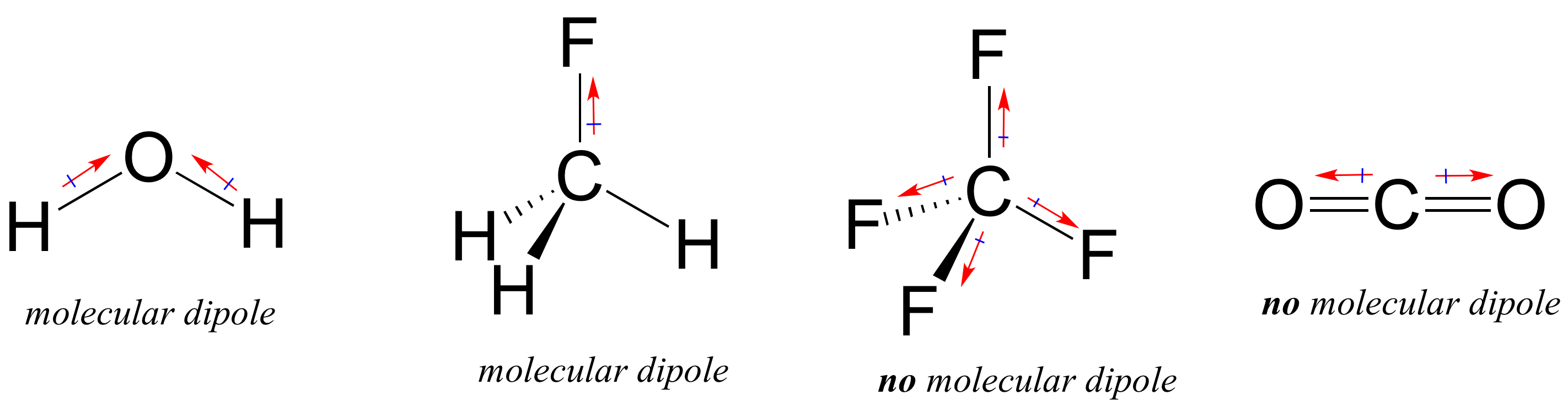
From www.chemistrylearner.com
Dipoledipole Forces Definition and Examples Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From chem.libretexts.org
3.4 Bond Polarity Chemistry LibreTexts Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From mydigitalkemistry.com
Hydrogen Bonding Definition ,Examples and Types Digital Kemistry Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From saylordotorg.github.io
Intermolecular Forces Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.youtube.com
Hydrogen Bonding And Dipole MomentChemical BondingClass 11 chapter 4 Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.chemistrysteps.com
Dipoledipole, London Dispersion and Hydrogen Bonding Interactions Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.chemistrysteps.com
Dipoledipole, London Dispersion and Hydrogen Bonding Interactions Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From sites.google.com
Bond Polarity/ Dipoles Chemical Bonding Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.chemistrysteps.com
Dipoledipole, London Dispersion and Hydrogen Bonding Interactions Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.slideserve.com
PPT Ch 10 PowerPoint Presentation, free download ID3538935 Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From mungfali.com
Hydrogen Bonding Vs Dipole Dipole Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.studypool.com
SOLUTION Intermolecular forces dipole dipole interaction hydrogen Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.slideteam.net
Dipole Dipole Hydrogen Bonding Ppt PowerPoint Presentation Infographic Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.youtube.com
Hydrogen Bonding, Dipole Dipole Ion Dipole Forces Strong Intermolecular Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From hillhouse4design.com
dipole dipole bond examples Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From www.slideserve.com
PPT Six Types 1) Dipole Dipole, 2) HydrogenBonding, 3) London Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.slideserve.com
PPT To learn about dipoledipole, hydrogen bonding and London Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From www.studypool.com
SOLUTION Intermolecular forces dipole dipole interaction hydrogen Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From hacfoot.weebly.com
Ion bonding hydrogen bonding dipole dipole hacfoot Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From study.com
Hydrogen Bonding, DipoleDipole & IonDipole Forces Strong Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From www.youtube.com
Dipole Dipole Interactions and Hydrogen Bonding! YouTube Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From www.science.org
An improved bound on the electron’s electric dipole moment Science Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From www.dreamstime.com
Vector Illustration of Hydrogen Bonding Chemical Bond Dipoledipole Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From www.youtube.com
Intermolecular forces Hydrogen bonding and Permanent dipoledipole Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From studiousguy.com
Intermolecular Force Types and Examples StudiousGuy Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.science-revision.co.uk
Dipoledipole forces Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.slideserve.com
PPT The chemical nature of cells PowerPoint Presentation, free Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.differencebetween.com
What is the Difference Between Dipole Dipole Interactions and Hydrogen Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.slideserve.com
PPT To learn about dipoledipole, hydrogen bonding and London Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From www.slideserve.com
PPT DipoleDipole Interactions PowerPoint Presentation, free download Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.chemistrysteps.com
Dipoledipole, London Dispersion and Hydrogen Bonding Interactions Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.
From evulpo.com
Hydrogen bonding Chemistry Explanation & Exercises evulpo Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From www.youtube.com
R Chemistry 06.02 Intermolecular Forces II DipoleDipole and Electric Dipole Hydrogen Bonding energy dissipation in water is very fast and more efficient than in many other liquids. This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. Electric Dipole Hydrogen Bonding.
From coggle.it
CHEMICAL BONDS The electrons that participated in this bonding are… Electric Dipole Hydrogen Bonding This behavior is commonly attributed to the intermolecular interactions associated with hydrogen bonding. energy dissipation in water is very fast and more efficient than in many other liquids. Electric Dipole Hydrogen Bonding.