Determining Heat Capacity Of A Calorimeter Lab Report . Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. The heat of reaction is the. By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. You can find specific heat capacity for different substances in table 5.2.1 on this page. Δtδt will determine the sign of the heat of the reaction (qq).
from www.studocu.com
You can find specific heat capacity for different substances in table 5.2.1 on this page. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. The heat of reaction is the. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Δtδt will determine the sign of the heat of the reaction (qq).
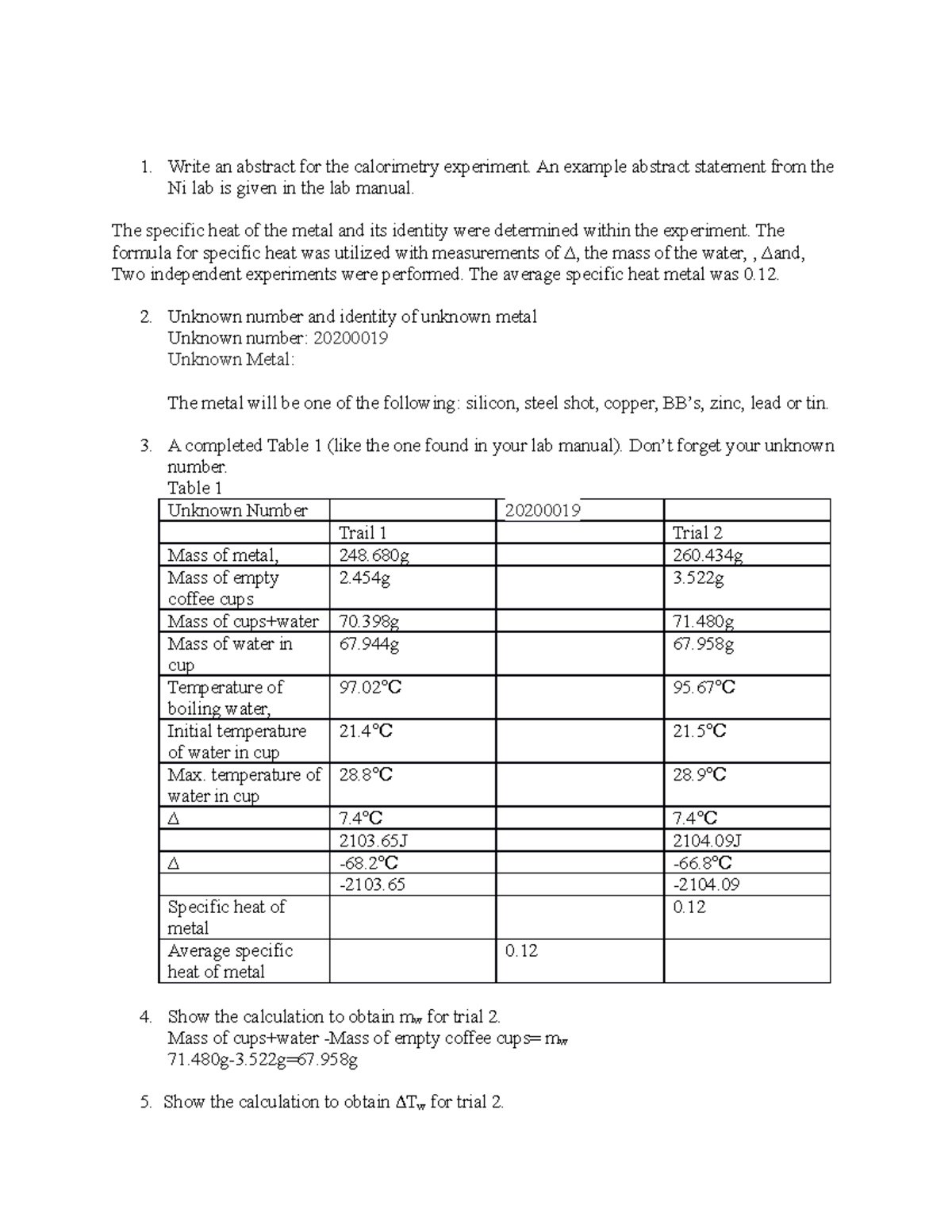
Calorimetry Lab Report Write an abstract for the calorimetry
Determining Heat Capacity Of A Calorimeter Lab Report Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The heat of reaction is the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Δtδt will determine the sign of the heat of the reaction (qq). To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. You can find specific heat capacity for different substances in table 5.2.1 on this page. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats.
From chemistrytalk.org
Calorimetry ChemTalk Determining Heat Capacity Of A Calorimeter Lab Report To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. The heat of reaction is the. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Δtδt will determine the sign of the heat. Determining Heat Capacity Of A Calorimeter Lab Report.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Determining Heat Capacity Of A Calorimeter Lab Report The heat of reaction is the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. You can find specific heat capacity for different substances in table 5.2.1 on this page. In this experiment you will heat a known mass of a metal to a known temperature and then transfer. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Determining Heat Capacity Of A Calorimeter Lab Report Δtδt will determine the sign of the heat of the reaction (qq). This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. To determine the. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. Δtδt will determine the sign of the heat of the reaction (qq). The heat of reaction is the. This experiment. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. You can find specific heat capacity for different substances in table 5.2.1 on this page. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. Abstract the main purpose of. Determining Heat Capacity Of A Calorimeter Lab Report.
From pdfprof.com
experiment 25 calorimetry lab report Determining Heat Capacity Of A Calorimeter Lab Report By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. In this experiment you will heat a. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studypool.com
SOLUTION Heat capacity of calorimeter and enthalpy of neutralization Determining Heat Capacity Of A Calorimeter Lab Report By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. The heat of reaction is the. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Determining Heat Capacity Of A Calorimeter Lab Report.
From studylib.net
Student instructions and lab report Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Determining Heat Capacity Of A Calorimeter Lab Report The heat of reaction is the. Δtδt will determine the sign of the heat of the reaction (qq). You can find specific heat capacity for different substances in table 5.2.1 on this page. Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. In this experiment you will heat a known mass. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Determining Heat Capacity Of A Calorimeter Lab Report By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. Δtδt will determine the sign of the. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.chegg.com
Solved CHEMISTRY DETERMINING HEAT CAPACITY OF A CALORIMETER Determining Heat Capacity Of A Calorimeter Lab Report By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. You can find specific heat capacity for different substances in table 5.2.1 on this page. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy,. Determining Heat Capacity Of A Calorimeter Lab Report.
From studylib.net
Calorimetry Lab Report Problem To experimentally determine the Determining Heat Capacity Of A Calorimeter Lab Report Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Δtδt will determine the sign of the heat of the reaction (qq). You can find specific heat capacity for different substances in table 5.2.1 on this page. In this experiment you will heat a known mass of a metal to. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Determining Heat Capacity Of A Calorimeter Lab Report To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. Δtδt will determine the sign of the heat of the reaction (qq). This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. In this experiment you will. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. The heat of reaction is the. This experiment allowed to determine and practice the theory of principles of calorimeter, which. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Determining Heat Capacity Of A Calorimeter Lab Report Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.pdfprof.com
determination of calorimeter constant lab report Determining Heat Capacity Of A Calorimeter Lab Report To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. You can find specific heat capacity for different substances in table 5.2.1 on this page. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. Abstract the. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Determining Heat Capacity Of A Calorimeter Lab Report The heat of reaction is the. Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. You can find specific heat capacity for different substances in table 5.2.1 on this. Determining Heat Capacity Of A Calorimeter Lab Report.
From studylib.net
LAB 3 SPECIFIC HEAT CAPACITY (method of Determining Heat Capacity Of A Calorimeter Lab Report The heat of reaction is the. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. By making a similar heat exchange measurement in. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Determining Heat Capacity Of A Calorimeter Lab Report In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. By making a similar heat exchange measurement in which we mix mh grams of. Determining Heat Capacity Of A Calorimeter Lab Report.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. The heat of reaction is the. You can find specific heat capacity for different substances in table 5.2.1 on this page. Δtδt will determine the sign of the heat of the reaction (qq). Abstract the main purpose of the calorimetry experiment is. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Determining Heat Capacity Of A Calorimeter Lab Report Δtδt will determine the sign of the heat of the reaction (qq). To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.scribd.com
Lab Report 1 Calorimetry Specific Heat Capacities of Metals Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at. Determining Heat Capacity Of A Calorimeter Lab Report.
From studylib.net
06.03 Calorimetry Lab Report Determining Heat Capacity Of A Calorimeter Lab Report In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studypool.com
SOLUTION Heat capacity of calorimeter and enthalpy of neutralization Determining Heat Capacity Of A Calorimeter Lab Report Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. You can find specific heat capacity for different substances in table 5.2.1 on this page. Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. Δtδt will determine the sign of the. Determining Heat Capacity Of A Calorimeter Lab Report.
From byjus.com
To Determine Specific Heat Capacity Of A Given Solid Physics Practical Determining Heat Capacity Of A Calorimeter Lab Report By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. The heat of reaction is the. Δtδt will determine the sign of the heat of the reaction (qq). You can find specific heat capacity for different substances in. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studocu.com
Procedures IN Determining THE HEAT Capacity OF A Calorimeter A Determining Heat Capacity Of A Calorimeter Lab Report You can find specific heat capacity for different substances in table 5.2.1 on this page. The heat of reaction is the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Determining Heat Capacity Of A Calorimeter Lab Report You can find specific heat capacity for different substances in table 5.2.1 on this page. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. The heat of reaction is the. Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat.. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.pdfprof.com
experiment 25 calorimetry lab report Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine. Determining Heat Capacity Of A Calorimeter Lab Report.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Determining Heat Capacity Of A Calorimeter Lab Report Δtδt will determine the sign of the heat of the reaction (qq). Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. The heat of reaction is the. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that.. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.scribd.com
Determining Specific Heat Capacities through Calorimetry Experiments Determining Heat Capacity Of A Calorimeter Lab Report Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the reaction. This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Determining Heat Capacity Of A Calorimeter Lab Report Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Δtδt will determine the sign of the heat of the reaction (qq). This experiment allowed to determine and practice the theory of principles of calorimeter, which is an apparatus used to determine heats. You can find specific heat capacity for. Determining Heat Capacity Of A Calorimeter Lab Report.
From studylib.net
Calorimetry Lab Specific Heat Capacity Determining Heat Capacity Of A Calorimeter Lab Report In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Δtδt will determine the sign of the heat of the reaction (qq). The heat of reaction is the. By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.scribd.com
Lab Report CHM02 CO3 Virtual Lab Determining Heat Capacity of A Determining Heat Capacity Of A Calorimeter Lab Report By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. You can find specific. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Determining Heat Capacity Of A Calorimeter Lab Report Through the use of a calibration sample of known combustion value (often benzoic acid, including here), the heat. By making a similar heat exchange measurement in which we mix mh grams of hot water, initially at temperature th, with mc grams of water initially at a lower temperature tc (perhaps. To determine the heat capacity of the calorimeter, a solution. Determining Heat Capacity Of A Calorimeter Lab Report.
From www.numerade.com
SOLVEDREPORT SHEET Heat of Neutralization EXPERIMENT 12 Heat Capacity Determining Heat Capacity Of A Calorimeter Lab Report You can find specific heat capacity for different substances in table 5.2.1 on this page. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. To determine the heat capacity of the calorimeter, a solution of hydrochloric acid was standardized and the temperature change from the. Determining Heat Capacity Of A Calorimeter Lab Report.