Medical Device Classification With Examples . Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Class i, class iia, class iib, and class iii medical devices. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Find out the regulatory requirements, product codes, and pathways to market for each device class. Find out the examples, requirements, and methods for. Fda, european commission, and health canada based on risk, intended use, and indications for use. Class i, ii, and iii. Learn how to classify your medical device by u.s.
from smartdataweek.com
Learn how to classify your medical device by u.s. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: Find out the examples, requirements, and methods for. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Class i, class iia, class iib, and class iii medical devices. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Class i, ii, and iii. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes:
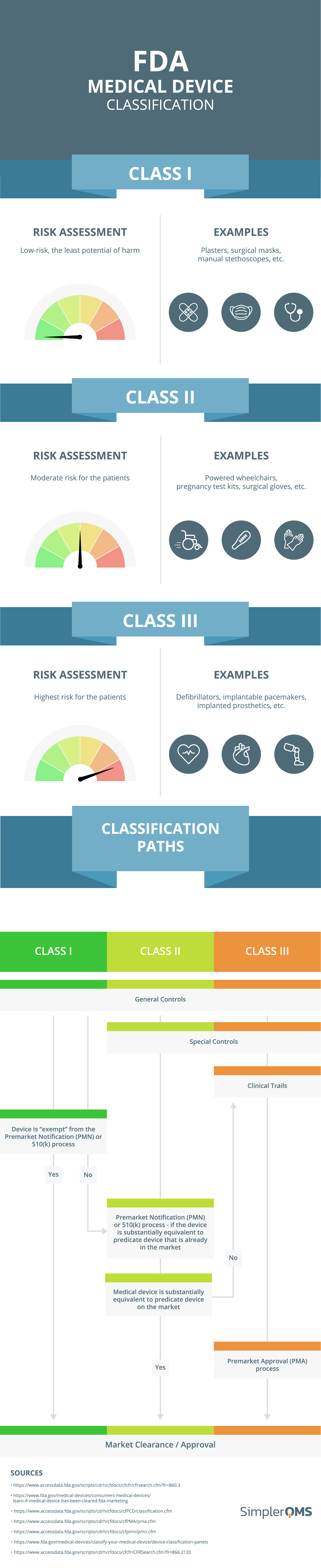
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024)
Medical Device Classification With Examples Class i, class iia, class iib, and class iii medical devices. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Class i, ii, and iii. Learn how to classify your medical device by u.s. Fda, european commission, and health canada based on risk, intended use, and indications for use. The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Find out the examples, requirements, and methods for. Class i, class iia, class iib, and class iii medical devices. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Find out the regulatory requirements, product codes, and pathways to market for each device class. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety:
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification With Examples Fda, european commission, and health canada based on risk, intended use, and indications for use. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Find out the examples, requirements, and methods for. Class i, class iia, class iib, and class iii medical devices. The european union medical device. Medical Device Classification With Examples.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification With Examples Learn how to classify your medical device by u.s. Find out the examples, requirements, and methods for. Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Class i, class iia, class iib, and class iii medical devices. The european union medical device regulation (eu mdr) categorizes medical devices into. Medical Device Classification With Examples.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification With Examples Fda, european commission, and health canada based on risk, intended use, and indications for use. Class i, ii, and iii. Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Class i, class iia, class iib, and class iii medical devices. Learn how to classify your medical device by u.s.. Medical Device Classification With Examples.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Medical Device Classification With Examples Fda, european commission, and health canada based on risk, intended use, and indications for use. Class i, class iia, class iib, and class iii medical devices. Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Learn how to classify medical devices according to the eu medical device regulation (mdr). Medical Device Classification With Examples.
From smartdataweek.com
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024) Medical Device Classification With Examples Learn how medical devices are classified into four classes based on their risks and requirements in the eu. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Class i, class. Medical Device Classification With Examples.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Device Classification With Examples Class i, ii, and iii. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Learn. Medical Device Classification With Examples.
From www.pacificbridgemedical.com
Device Classification in India Infographic Medical Device Classification With Examples Class i, ii, and iii. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Fda, european commission, and health canada based on risk, intended use, and indications for use. Find out the. Medical Device Classification With Examples.
From mavink.com
Types Of Medical Devices List Medical Device Classification With Examples Learn how to classify your medical device by u.s. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Class i, ii, and iii. Find out the regulatory requirements, product codes, and pathways to market for each device class. Learn how the fda regulates medical devices based on their risk profile and. Medical Device Classification With Examples.
From www.medicalmicromolding.com
UK Medical Device Classification Medical Device Classification With Examples Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: Class i, class iia,. Medical Device Classification With Examples.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Classification With Examples Learn how to classify your medical device by u.s. Fda, european commission, and health canada based on risk, intended use, and indications for use. Find out the examples, requirements, and methods for. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: Find out the regulatory requirements, product codes, and pathways to market. Medical Device Classification With Examples.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Classification With Examples Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Learn how the fda. Medical Device Classification With Examples.
From omcmedical.com
EU Classification of Medical Devices OMC Medical Medical Device Classification With Examples Find out the regulatory requirements, product codes, and pathways to market for each device class. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Fda, european commission, and health canada based on risk,. Medical Device Classification With Examples.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification With Examples Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Class i, class iia, class iib, and class iii medical devices. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Learn how the fda categorizes medical devices into three classes based. Medical Device Classification With Examples.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Classification With Examples The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Find out the regulatory requirements, product codes, and pathways to market for each device class. Find out the examples, requirements, and methods for. Class i, ii, and iii. Learn how the fda categorizes medical devices into three classes based on their risk to patient. Medical Device Classification With Examples.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Classification With Examples Class i, ii, and iii. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Find out the regulatory requirements, product codes, and pathways to market for each device class. Find. Medical Device Classification With Examples.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Classification With Examples Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Learn how to classify your medical device by u.s. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: The european union medical device regulation (eu mdr) categorizes medical devices into one of. Medical Device Classification With Examples.
From www.researchgate.net
Medical Device Classification a Download Scientific Diagram Medical Device Classification With Examples Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: Class i, class iia, class iib, and class iii medical devices. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Find out the examples, requirements, and methods for. Find out the regulatory requirements, product. Medical Device Classification With Examples.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Classification With Examples Find out the examples, requirements, and methods for. Learn how to classify your medical device by u.s. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Learn how the fda regulates medical devices. Medical Device Classification With Examples.
From gbu-taganskij.ru
Complete Guide Medical Device Classification EU MDR (Free, 58 OFF Medical Device Classification With Examples Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: Find out the examples, requirements, and methods for. Class i, ii, and iii. Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Class i, class iia, class iib, and class iii medical. Medical Device Classification With Examples.
From www.arenasolutions.com
How to Classify Your Medical Device Under the EU MDR and IVDR Arena Medical Device Classification With Examples Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Fda, european commission, and health canada based on risk, intended use, and indications for use. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: The european union medical device regulation (eu mdr) categorizes medical. Medical Device Classification With Examples.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification With Examples Fda, european commission, and health canada based on risk, intended use, and indications for use. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The european union medical device regulation (eu mdr) categorizes. Medical Device Classification With Examples.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Classification With Examples Fda, european commission, and health canada based on risk, intended use, and indications for use. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Find out the regulatory requirements, product codes, and pathways to market for each device class. Learn how medical devices are classified into four classes. Medical Device Classification With Examples.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device Classification With Examples Find out the examples, requirements, and methods for. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Find out the regulatory requirements, product codes, and pathways to market for each device class. Class i, ii, and iii. Learn how the fda regulates medical devices based on their risk profile and assigns. Medical Device Classification With Examples.
From www.presentationeze.com
FDA medical device classification PresentationEZE Medical Device Classification With Examples Class i, class iia, class iib, and class iii medical devices. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Find out the regulatory requirements, product codes, and pathways to market for each device class. Find out the examples, requirements, and methods for. Learn how to classify medical devices according to. Medical Device Classification With Examples.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification With Examples Class i, ii, and iii. The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Fda, european commission, and health canada based on risk, intended use, and indications for use. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Learn how the fda categorizes medical. Medical Device Classification With Examples.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Classification With Examples Find out the regulatory requirements, product codes, and pathways to market for each device class. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Learn how to classify medical devices according to the eu medical device. Medical Device Classification With Examples.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Medical Device Classification With Examples Class i, class iia, class iib, and class iii medical devices. The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Fda, european commission, and health canada based on risk, intended use, and indications. Medical Device Classification With Examples.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Classification With Examples Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Class i, ii, and iii. Find out the regulatory requirements, product codes, and pathways to market for each device class. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: The food. Medical Device Classification With Examples.
From www.arenasolutions.com
Why Medical Device Classification Matters Arena Medical Device Classification With Examples The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Class i, class iia, class iib, and class iii medical devices. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types. Medical Device Classification With Examples.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Device Classification With Examples Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Learn how to classify medical devices according to the eu medical device regulation (mdr) based on their risk and regulatory control. Find out the examples, requirements, and methods for. Find out the regulatory requirements, product codes, and pathways to market for each. Medical Device Classification With Examples.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Classification With Examples Class i, class iia, class iib, and class iii medical devices. Class i, ii, and iii. Fda, european commission, and health canada based on risk, intended use, and indications for use. Learn how to classify your medical device by u.s. Learn how the fda categorizes medical devices into three classes based on their risk to patient safety: The european union. Medical Device Classification With Examples.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Classification With Examples Class i, class iia, class iib, and class iii medical devices. Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Fda, european commission, and health canada based on risk, intended use,. Medical Device Classification With Examples.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Classification With Examples Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Find out the regulatory requirements, product codes, and pathways to market for each device class. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Learn how the fda categorizes medical devices. Medical Device Classification With Examples.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Classification With Examples Learn how the fda regulates medical devices based on their risk profile and assigns them to one of three classes: Learn how to classify your medical device by u.s. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Find out the regulatory requirements, product codes, and pathways to market for each. Medical Device Classification With Examples.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Classification With Examples Class i, class iia, class iib, and class iii medical devices. Learn how medical devices are classified into four classes based on their risks and requirements in the eu. Learn how to classify your medical device by u.s. The european union medical device regulation (eu mdr) categorizes medical devices into one of four classes: Learn how to classify medical devices. Medical Device Classification With Examples.