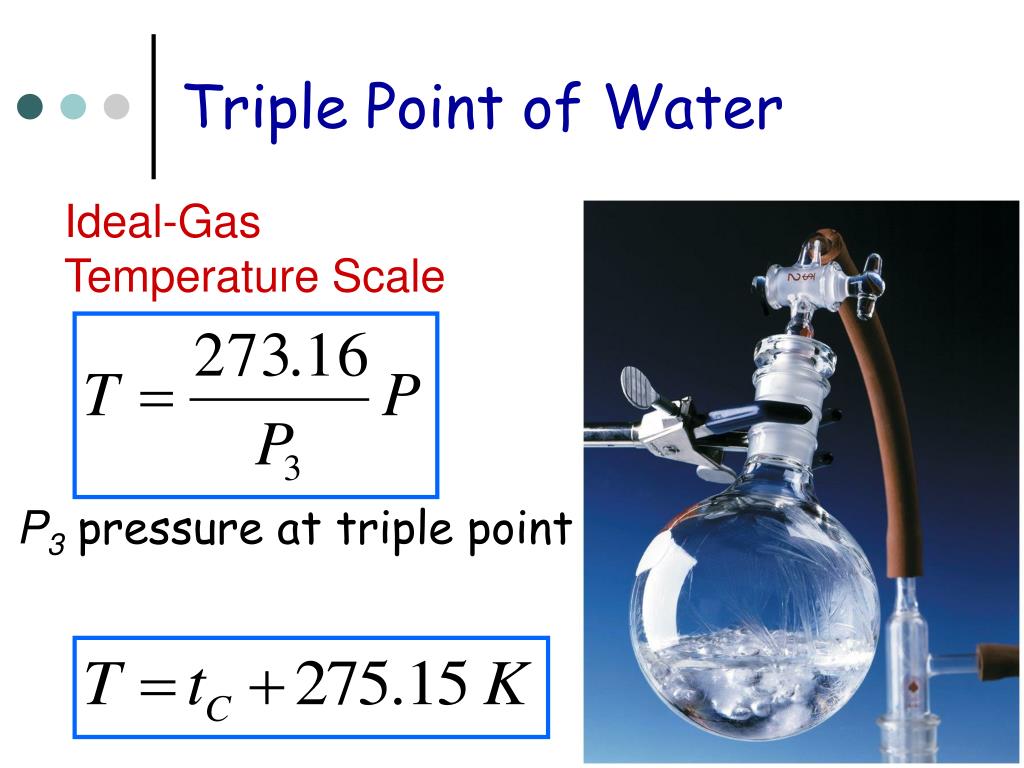
What Is A Triple Point Of Water . Note the triple point may include more than one solid phase if a specific substance has polymorphs. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. The triple point of water is the unique condition at which water can coexist in all three phases: The dashed green line gives the anomalous behavior of water. One example of a triple point in nature is the triple point of water. 43 rows the solid green line applies to most substances; Solid (ice), liquid (water), and gas (water vapor) at a. At this point, water can exist in all three states: The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature.
from www.slideserve.com
The triple point of water is the unique condition at which water can coexist in all three phases: One example of a triple point in nature is the triple point of water. 43 rows the solid green line applies to most substances; The triple point for water is at 0.01 degree celsius at 4.56 mm hg. Solid (ice), liquid (water), and gas (water vapor) at a. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. Note the triple point may include more than one solid phase if a specific substance has polymorphs. The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. At this point, water can exist in all three states: The dashed green line gives the anomalous behavior of water.
PPT Chapter 17 Theory of Gases PowerPoint Presentation, free
What Is A Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. 43 rows the solid green line applies to most substances; The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. One example of a triple point in nature is the triple point of water. At this point, water can exist in all three states: The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. Note the triple point may include more than one solid phase if a specific substance has polymorphs. The dashed green line gives the anomalous behavior of water. The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice), liquid (water), and gas (water vapor) at a.
From jakobeewtcoller.blogspot.com
Triple Point of Water JakobeewtColler What Is A Triple Point Of Water Note the triple point may include more than one solid phase if a specific substance has polymorphs. The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. The dashed green line gives the anomalous behavior of water. At this point, water can exist in all three states: The. What Is A Triple Point Of Water.
From wireblueprint.com
Exploring the Triple Point of Water Understanding the Phase Diagram What Is A Triple Point Of Water The triple point for water is at 0.01 degree celsius at 4.56 mm hg. At this point, water can exist in all three states: The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. The dashed green line gives the anomalous behavior of water. One example of a. What Is A Triple Point Of Water.
From www.doubtnut.com
Triple point of water corresponds to What Is A Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. One example of a triple point in nature is the triple point of water. The triple point of water is the unique condition at which water can coexist. What Is A Triple Point Of Water.
From www.toppr.com
Two absolute scale X and Y assigned numerical values 200 and 450 to the What Is A Triple Point Of Water Note the triple point may include more than one solid phase if a specific substance has polymorphs. One example of a triple point in nature is the triple point of water. The dashed green line gives the anomalous behavior of water. At this point, water can exist in all three states: The triple point of water is the unique condition. What Is A Triple Point Of Water.
From www.chemix-chemistry-software.com
Water Triple point Critical point Phase diagram What Is A Triple Point Of Water At this point, water can exist in all three states: One example of a triple point in nature is the triple point of water. The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice), liquid (water), and gas (water vapor) at a. The term triple point refers to the point. What Is A Triple Point Of Water.
From www.youtube.com
Triple Point Phase Diagram of water YouTube What Is A Triple Point Of Water One example of a triple point in nature is the triple point of water. Note the triple point may include more than one solid phase if a specific substance has polymorphs. The triple point for water is at 0.01 degree celsius at 4.56 mm hg. 43 rows the solid green line applies to most substances; The dashed green line gives. What Is A Triple Point Of Water.
From www.slideserve.com
PPT Chapter 17 Theory of Gases PowerPoint Presentation, free What Is A Triple Point Of Water 43 rows the solid green line applies to most substances; The triple point of water is the unique condition at which water can coexist in all three phases: The triple point for water is at 0.01 degree celsius at 4.56 mm hg. At this point, water can exist in all three states: The triple point of water is a fixed. What Is A Triple Point Of Water.
From www.youtube.com
Triple Point of Water YouTube What Is A Triple Point Of Water The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The dashed green line gives the anomalous behavior of water. 43 rows the solid green line applies to most substances; At this point, water can exist in all three states: One example of a triple point in nature is the triple point of water. The triple. What Is A Triple Point Of Water.
From www.youtube.com
CONCEPT OF TRIPLE POINT TRIPLE POINT OF WATER 💧 YouTube What Is A Triple Point Of Water The triple point for water is at 0.01 degree celsius at 4.56 mm hg. One example of a triple point in nature is the triple point of water. The triple point of water is the unique condition at which water can coexist in all three phases: The triple point of water is a fixed quantity, used to define other triple. What Is A Triple Point Of Water.
From www.youtube.com
Triple Point of Water YouTube What Is A Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. 43 rows the solid green line applies to most substances; One example of a triple point in nature is the triple point of water. The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. The triple point of. What Is A Triple Point Of Water.
From www.youtube.com
PCAT Phase Diagram for Water (Triple Point, Critical Point What Is A Triple Point Of Water The dashed green line gives the anomalous behavior of water. Solid (ice), liquid (water), and gas (water vapor) at a. 43 rows the solid green line applies to most substances; The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The term triple point refers to the point at which solid, liquid, and vapor phases coexist. What Is A Triple Point Of Water.
From sciencenotes.org
Triple Point Definition Triple Point of Water What Is A Triple Point Of Water 43 rows the solid green line applies to most substances; Solid (ice), liquid (water), and gas (water vapor) at a. One example of a triple point in nature is the triple point of water. Note the triple point may include more than one solid phase if a specific substance has polymorphs. The triple point of water is the unique condition. What Is A Triple Point Of Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Is A Triple Point Of Water The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. At this point, water can exist in all three states: The dashed green line gives the anomalous behavior of water. One example of a triple point in nature is the triple point of water. The term triple point. What Is A Triple Point Of Water.
From www.zigya.com
The Triple Point of Water Coexistence of Solid Liquid and Gas What Is A Triple Point Of Water The triple point for water is at 0.01 degree celsius at 4.56 mm hg. 43 rows the solid green line applies to most substances; Solid (ice), liquid (water), and gas (water vapor) at a. At this point, water can exist in all three states: Note the triple point may include more than one solid phase if a specific substance has. What Is A Triple Point Of Water.
From isotech.co.uk
JarrettIsotech Water Triple Point Cells Isotech What Is A Triple Point Of Water At this point, water can exist in all three states: The dashed green line gives the anomalous behavior of water. One example of a triple point in nature is the triple point of water. The triple point of water is the unique condition at which water can coexist in all three phases: The term triple point refers to the point. What Is A Triple Point Of Water.
From wirecrafted.com
Understanding the Triple Point of Water A Crucial Phase Diagram What Is A Triple Point Of Water The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. The triple point of water is the unique condition at which water can coexist in all three phases: The dashed green line. What Is A Triple Point Of Water.
From www.thoughtco.com
Triple Point Definition and Example (Chemistry) What Is A Triple Point Of Water 43 rows the solid green line applies to most substances; The triple point of water is the unique condition at which water can coexist in all three phases: One example of a triple point in nature is the triple point of water. The dashed green line gives the anomalous behavior of water. The triple point for water is at 0.01. What Is A Triple Point Of Water.
From www.youtube.com
The Triple Point of Water An Animation to Explain YouTube What Is A Triple Point Of Water The triple point of water is the unique condition at which water can coexist in all three phases: At this point, water can exist in all three states: Note the triple point may include more than one solid phase if a specific substance has polymorphs. The triple point for water is at 0.01 degree celsius at 4.56 mm hg. One. What Is A Triple Point Of Water.
From enginerileyseemless.z14.web.core.windows.net
Phase Diagram Of Water Triple Point What Is A Triple Point Of Water At this point, water can exist in all three states: The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice), liquid (water), and gas (water vapor) at a. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature. What Is A Triple Point Of Water.
From byjus.com
triple point of water What Is A Triple Point Of Water 43 rows the solid green line applies to most substances; One example of a triple point in nature is the triple point of water. At this point, water can exist in all three states: The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The term triple point refers to the point at which solid, liquid,. What Is A Triple Point Of Water.
From wirecrafted.com
Understanding the Triple Point of Water A Crucial Phase Diagram What Is A Triple Point Of Water Note the triple point may include more than one solid phase if a specific substance has polymorphs. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. The triple point of water is the unique condition at which water can coexist in all three phases: One. What Is A Triple Point Of Water.
From www.youtube.com
ADT878 Triple Point of Water How To YouTube What Is A Triple Point Of Water One example of a triple point in nature is the triple point of water. Solid (ice), liquid (water), and gas (water vapor) at a. Note the triple point may include more than one solid phase if a specific substance has polymorphs. 43 rows the solid green line applies to most substances; The dashed green line gives the anomalous behavior of. What Is A Triple Point Of Water.
From www.pondengineering.com
K29A/B Triple Point of Water Cell — Pond Engineering Laboratories, Inc. What Is A Triple Point Of Water The dashed green line gives the anomalous behavior of water. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. At this point, water can exist in all three states: Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of water is. What Is A Triple Point Of Water.
From www.researchgate.net
Phase diagram with a triple point O of water analogy. Download What Is A Triple Point Of Water The dashed green line gives the anomalous behavior of water. Note the triple point may include more than one solid phase if a specific substance has polymorphs. One example of a triple point in nature is the triple point of water. The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The triple point of water. What Is A Triple Point Of Water.
From naeye.net
Triple Point of Water The Temperature Where All Three Phases Coexist What Is A Triple Point Of Water At this point, water can exist in all three states: One example of a triple point in nature is the triple point of water. The triple point of water is the unique condition at which water can coexist in all three phases: The dashed green line gives the anomalous behavior of water. The triple point of water is a fixed. What Is A Triple Point Of Water.
From wireblueprint.com
Exploring the Triple Point of Water Understanding the Phase Diagram What Is A Triple Point Of Water The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. The triple point of water is the unique condition at which water can coexist in all three phases: The dashed green line gives the anomalous behavior of water. Note the triple point may include more than. What Is A Triple Point Of Water.
From www.youtube.com
Triple point of water YouTube What Is A Triple Point Of Water 43 rows the solid green line applies to most substances; One example of a triple point in nature is the triple point of water. The triple point of water is the unique condition at which water can coexist in all three phases: The dashed green line gives the anomalous behavior of water. The term triple point refers to the point. What Is A Triple Point Of Water.
From www.researchgate.net
Triple point of water analogy. intensive variables critical What Is A Triple Point Of Water The dashed green line gives the anomalous behavior of water. The triple point of water is the unique condition at which water can coexist in all three phases: Note the triple point may include more than one solid phase if a specific substance has polymorphs. Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of water. What Is A Triple Point Of Water.
From www.nuclear-power.com
Triple Point of Water Definition & Value What Is A Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. One example of a triple point in nature is the triple point of water. The dashed green line gives the anomalous behavior of water. Note the triple point may include more than one solid phase if a specific substance has polymorphs. The triple point of water is the unique condition. What Is A Triple Point Of Water.
From klasmeier.engineer
Water Triple Point Thomas Klasmeier What Is A Triple Point Of Water At this point, water can exist in all three states: The dashed green line gives the anomalous behavior of water. Note the triple point may include more than one solid phase if a specific substance has polymorphs. One example of a triple point in nature is the triple point of water. The term triple point refers to the point at. What Is A Triple Point Of Water.
From wirecrafted.com
Understanding the Water Triple Point Diagram What Is A Triple Point Of Water One example of a triple point in nature is the triple point of water. The term triple point refers to the point at which solid, liquid, and vapor phases coexist in equilibrium at a given temperature and pressure. 43 rows the solid green line applies to most substances; The triple point of water is the unique condition at which water. What Is A Triple Point Of Water.
From www.researchgate.net
Phase diagram of water indicating the triple point [33] Download What Is A Triple Point Of Water The triple point of water is a fixed quantity, used to define other triple point values and the kelvin unit of temperature. The triple point for water is at 0.01 degree celsius at 4.56 mm hg. The dashed green line gives the anomalous behavior of water. Solid (ice), liquid (water), and gas (water vapor) at a. 43 rows the solid. What Is A Triple Point Of Water.
From curiophysics.com
Triple Point Of Water » Curio Physics What Is A Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. 43 rows the solid green line applies to most substances; The dashed green line gives the anomalous behavior of water. Note the triple point may include more than one solid phase if a specific substance has polymorphs. At this point, water can exist in all three states: One example of. What Is A Triple Point Of Water.
From www.youtube.com
Triple Point of Water The Calibration Constant YouTube What Is A Triple Point Of Water The triple point for water is at 0.01 degree celsius at 4.56 mm hg. One example of a triple point in nature is the triple point of water. The dashed green line gives the anomalous behavior of water. Note the triple point may include more than one solid phase if a specific substance has polymorphs. 43 rows the solid green. What Is A Triple Point Of Water.
From mungfali.com
Triple Point Phase Diagram What Is A Triple Point Of Water The triple point for water is at 0.01 degree celsius at 4.56 mm hg. Note the triple point may include more than one solid phase if a specific substance has polymorphs. One example of a triple point in nature is the triple point of water. 43 rows the solid green line applies to most substances; Solid (ice), liquid (water), and. What Is A Triple Point Of Water.