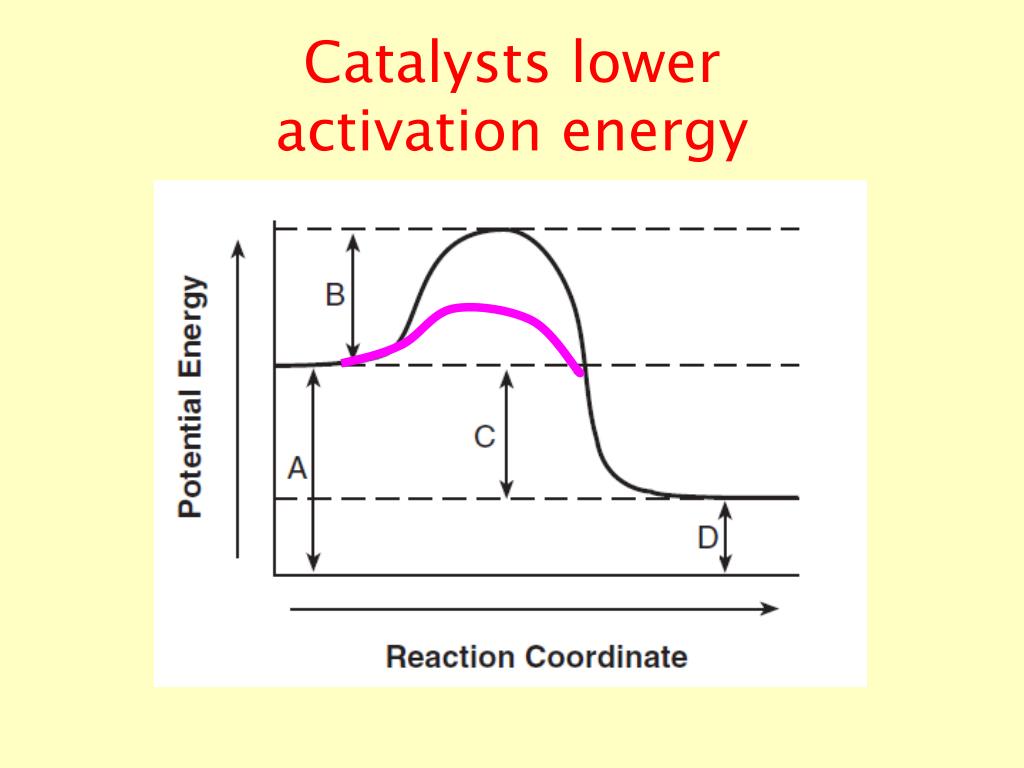
Catalysts Activation Energy . Instead they provide a new. Enzymes are examples of catalysts. Catalysts are not consumed by the. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts and activation energy. When a catalyst is used at 300 k, the rate increases one. One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. a catalyst lowers the activation energy of a chemical reaction. Catalysts do this by providing an alternative. the activation energy of a reaction is 19.0 kj/mol. catalysts increase the reaction rate by reducing the activation energy of the reaction.
from www.slideserve.com
the activation energy of a reaction is 19.0 kj/mol. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. When a catalyst is used at 300 k, the rate increases one. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. catalysts and activation energy. One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. a catalyst lowers the activation energy of a chemical reaction. Catalysts do this by providing an alternative. catalysts increase the reaction rate by reducing the activation energy of the reaction.
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free
Catalysts Activation Energy Catalysts do this by providing an alternative. When a catalyst is used at 300 k, the rate increases one. To increase the rate of a reaction you need to increase the number of successful collisions. a catalyst lowers the activation energy of a chemical reaction. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. catalysts and activation energy. One possible way of doing this. the activation energy of a reaction is 19.0 kj/mol. catalysts increase the reaction rate by reducing the activation energy of the reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Instead they provide a new. Catalysts do this by providing an alternative. Catalysts are not consumed by the. Enzymes are examples of catalysts.
From www.slideserve.com
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free Catalysts Activation Energy Catalysts are not consumed by the. a catalyst lowers the activation energy of a chemical reaction. One possible way of doing this. Enzymes are examples of catalysts. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. catalysts and activation energy. catalysts are defined as. Catalysts Activation Energy.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Catalysts Activation Energy catalysts increase the reaction rate by reducing the activation energy of the reaction. To increase the rate of a reaction you need to increase the number of successful collisions. When a catalyst is used at 300 k, the rate increases one. Instead they provide a new. Catalysts do this by providing an alternative. Catalysts are not consumed by the.. Catalysts Activation Energy.
From gradegorilla.com
Gradegorilla Chemistry Catalysts Activation Energy Instead they provide a new. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300 k, the rate increases one. catalysts increase the reaction rate by reducing the activation energy of the reaction. . Catalysts Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalysts Activation Energy catalysts and activation energy. Enzymes are examples of catalysts. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the activation energy of a reaction is 19.0 kj/mol.. Catalysts Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalysts Activation Energy When a catalyst is used at 300 k, the rate increases one. a catalyst lowers the activation energy of a chemical reaction. Instead they provide a new. catalysts and activation energy. One possible way of doing this. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in. Catalysts Activation Energy.
From www.savemyexams.com
Lowering of Activation Energy College Board AP Biology Revision Notes Catalysts Activation Energy Catalysts do this by providing an alternative. Catalysts are not consumed by the. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. a catalyst lowers the activation energy of. Catalysts Activation Energy.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalysts Activation Energy One possible way of doing this. Catalysts do this by providing an alternative. the activation energy of a reaction is 19.0 kj/mol. Instead they provide a new. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. Enzymes are examples of catalysts. catalysts are defined as. Catalysts Activation Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation ID1835084 Catalysts Activation Energy One possible way of doing this. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Enzymes are examples of catalysts. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. Catalysts do this by providing an alternative. catalysts. Catalysts Activation Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalysts Activation Energy catalysts and activation energy. Instead they provide a new. the activation energy of a reaction is 19.0 kj/mol. To increase the rate of a reaction you need to increase the number of successful collisions. a catalyst lowers the activation energy of a chemical reaction. One possible way of doing this. Catalysts do this by providing an alternative.. Catalysts Activation Energy.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Catalysts Activation Energy When a catalyst is used at 300 k, the rate increases one. One possible way of doing this. catalysts and activation energy. the activation energy of a reaction is 19.0 kj/mol. catalysts increase the reaction rate by reducing the activation energy of the reaction. To increase the rate of a reaction you need to increase the number. Catalysts Activation Energy.
From www.researchgate.net
Arrhenius plots of activation energy of N2O on the Catalysts Activation Energy Enzymes are examples of catalysts. Catalysts are not consumed by the. When a catalyst is used at 300 k, the rate increases one. Catalysts do this by providing an alternative. One possible way of doing this. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts are. Catalysts Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts Activation Energy Enzymes are examples of catalysts. When a catalyst is used at 300 k, the rate increases one. catalysts increase the reaction rate by reducing the activation energy of the reaction. Catalysts do this by providing an alternative. catalysts and activation energy. One possible way of doing this. the activation energy of a reaction is 19.0 kj/mol. . Catalysts Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalysts Activation Energy catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. Catalysts do this by providing an alternative. Instead they provide a new. catalysts are defined as. Catalysts Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalysts Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. the activation energy of a reaction is 19.0 kj/mol. One possible way of doing this. When a catalyst is used at 300 k, the rate increases one. To increase the rate of a reaction you need to increase the number of. Catalysts Activation Energy.
From www.sliderbase.com
Catalysis Presentation Chemistry Catalysts Activation Energy catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. catalysts increase the reaction rate by reducing the activation energy of the reaction. Catalysts are not consumed by the. One possible way of doing this. catalysts and activation energy. To increase the rate of a reaction. Catalysts Activation Energy.
From www.chim.lu
Activation energy and catalysis Catalysts Activation Energy catalysts and activation energy. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. One possible way of doing this. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts are not consumed by the. catalysts increase. Catalysts Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Catalysts Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts increase the reaction rate by reducing the activation energy of the reaction. One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts are not consumed by the.. Catalysts Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysts Activation Energy Instead they provide a new. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts increase the reaction rate by reducing the activation energy of the reaction. a catalyst. Catalysts Activation Energy.
From chem.libretexts.org
6.10 Energies, and Catalysts Chemistry LibreTexts Catalysts Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Enzymes are examples of catalysts. a catalyst lowers the activation energy of a chemical reaction. Catalysts do this by providing an alternative. When a catalyst is used at 300 k, the rate increases one. Catalysts are not consumed by the. . Catalysts Activation Energy.
From studyonline.blog
What Is Activation Energy? Definition and Examples Catalysts Activation Energy catalysts increase the reaction rate by reducing the activation energy of the reaction. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. When a catalyst is used at 300 k,. Catalysts Activation Energy.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalysts Activation Energy a catalyst lowers the activation energy of a chemical reaction. catalysts increase the reaction rate by reducing the activation energy of the reaction. the activation energy of a reaction is 19.0 kj/mol. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts increase the. Catalysts Activation Energy.
From www.tes.com
Catalysts and Activation Energy GCSE lesson (SC18c CC14c) Teaching Catalysts Activation Energy catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. catalysts increase the reaction rate by reducing the activation energy of the reaction. a catalyst lowers the activation energy of a chemical reaction. the activation energy of a reaction is 19.0 kj/mol. catalysts and. Catalysts Activation Energy.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Activation Energy the activation energy of a reaction is 19.0 kj/mol. Instead they provide a new. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. Catalysts do this by providing an alternative. catalysts are defined as substances that participate in a chemical reaction but are not changed. Catalysts Activation Energy.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Catalysts Activation Energy catalysts increase the reaction rate by reducing the activation energy of the reaction. Catalysts are not consumed by the. a catalyst lowers the activation energy of a chemical reaction. Catalysts do this by providing an alternative. Instead they provide a new. Enzymes are examples of catalysts. catalysts and activation energy. the activation energy of a reaction. Catalysts Activation Energy.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Catalysts Activation Energy catalysts increase the reaction rate by reducing the activation energy of the reaction. catalysts and activation energy. Catalysts do this by providing an alternative. the activation energy of a reaction is 19.0 kj/mol. Enzymes are examples of catalysts. Catalysts are not consumed by the. One possible way of doing this. Instead they provide a new. To increase. Catalysts Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Catalysts Activation Energy To increase the rate of a reaction you need to increase the number of successful collisions. catalysts and activation energy. Enzymes are examples of catalysts. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. a catalyst lowers the activation energy of a chemical reaction. Instead. Catalysts Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalysts Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Instead they provide a new. catalysts and activation energy. Enzymes are examples of catalysts. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. Catalysts are not consumed by. Catalysts Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts Activation Energy Enzymes are examples of catalysts. the activation energy of a reaction is 19.0 kj/mol. Instead they provide a new. When a catalyst is used at 300 k, the rate increases one. To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this. a catalyst lowers the activation. Catalysts Activation Energy.
From schematicdatavenin77.z5.web.core.windows.net
Energy Level Diagram Catalyst Catalysts Activation Energy catalysts increase the reaction rate by reducing the activation energy of the reaction. a catalyst lowers the activation energy of a chemical reaction. When a catalyst is used at 300 k, the rate increases one. To increase the rate of a reaction you need to increase the number of successful collisions. the activation energy of a reaction. Catalysts Activation Energy.
From www.pnnl.gov
Catalysis PNNL Catalysts Activation Energy To increase the rate of a reaction you need to increase the number of successful collisions. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. Enzymes are examples of catalysts. the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300. Catalysts Activation Energy.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalysts Activation Energy catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. the activation energy of a reaction is 19.0 kj/mol. One possible way of doing this. When a catalyst is used at 300 k, the rate increases one. Catalysts do this by providing an alternative. Enzymes are examples. Catalysts Activation Energy.
From www.researchgate.net
Activation energy for methane combustion with the two catalysts Catalysts Activation Energy catalysts increase the reaction rate by reducing the activation energy of the reaction. a catalyst lowers the activation energy of a chemical reaction. the activation energy of a reaction is 19.0 kj/mol. Catalysts do this by providing an alternative. Enzymes are examples of catalysts. catalysts increase the rates of reactions by providing a new mechanism that. Catalysts Activation Energy.
From 2012books.lardbucket.org
Catalysis Catalysts Activation Energy catalysts increase the reaction rate by reducing the activation energy of the reaction. a catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts and activation energy. catalysts increase the rates of reactions by providing a new mechanism. Catalysts Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Activation Energy Catalysts are not consumed by the. One possible way of doing this. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. the activation energy of a reaction is 19.0 kj/mol. catalysts are defined as substances that participate in a chemical reaction but are not changed. Catalysts Activation Energy.
From schematiccntrylivinjhfxe.z21.web.core.windows.net
Energy Diagram Catalyst Catalysts Activation Energy Enzymes are examples of catalysts. Catalysts do this by providing an alternative. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. One possible way of doing this. Catalysts are not consumed by the. catalysts and activation energy. To increase the rate of a reaction you need to increase the number. Catalysts Activation Energy.