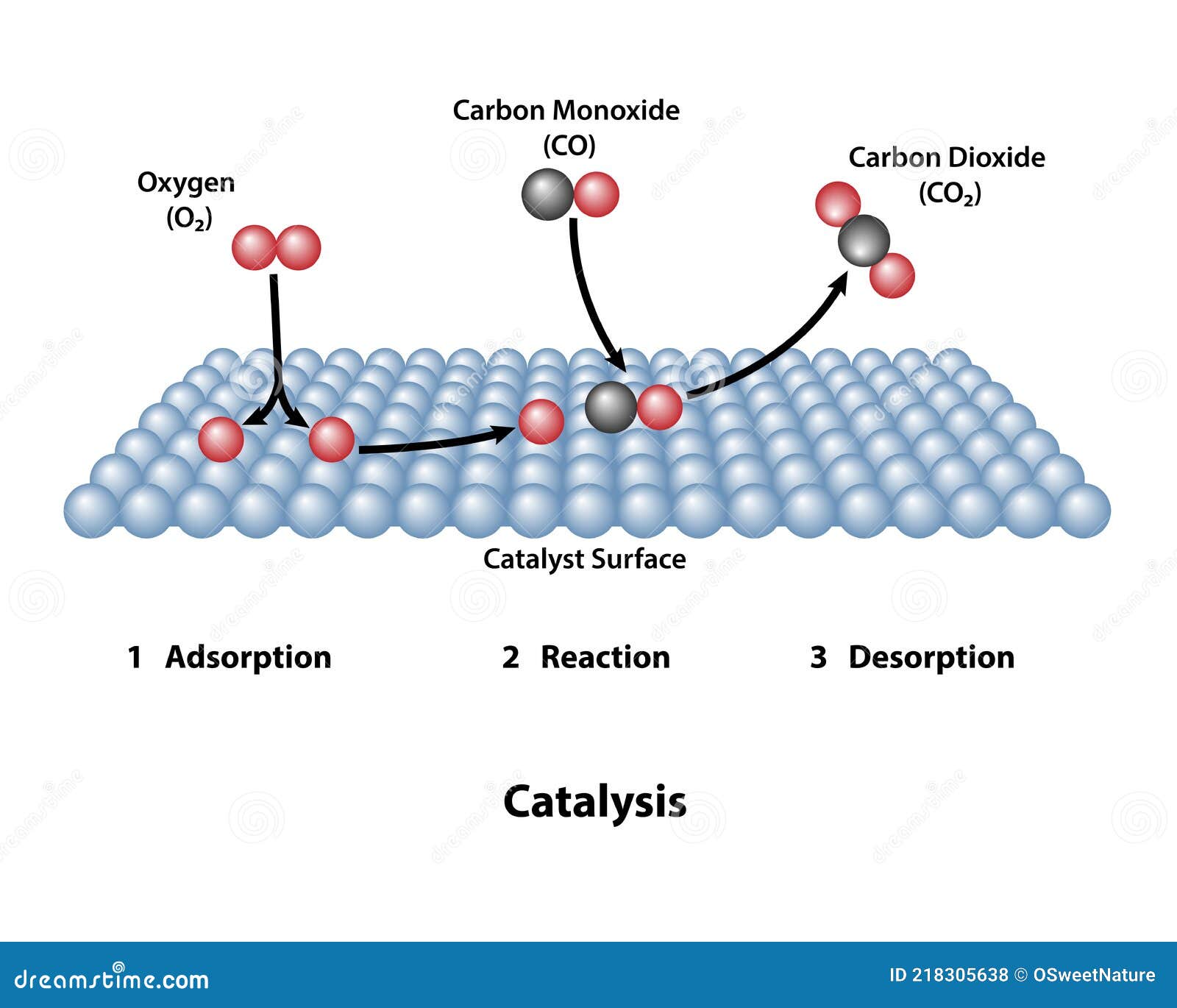
Catalyst Basic Examples . Enzymes are naturally occurring catalysts responsible for many. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. This lesson will give you a. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. There are four main types of catalysts: For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. Inorganic catalysts and organic catalysts. There are two main types of catalysts: A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
from www.dreamstime.com
Inorganic catalysts and organic catalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. There are four main types of catalysts: This lesson will give you a. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. There are two main types of catalysts: An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. Enzymes are naturally occurring catalysts responsible for many.
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of
Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. Inorganic catalysts and organic catalysts. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. Enzymes are naturally occurring catalysts responsible for many. There are two main types of catalysts: A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. There are four main types of catalysts: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This lesson will give you a. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Basic Examples There are four main types of catalysts: Inorganic catalysts and organic catalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. For example, a catalyst could cause a reaction between reactants to happen at a faster. Catalyst Basic Examples.
From ar.inspiredpencil.com
Catalyst Examples Catalyst Basic Examples There are two main types of catalysts: Enzymes are naturally occurring catalysts responsible for many. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. There are four main types of catalysts: Catalyst, in chemistry, any. Catalyst Basic Examples.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Basic Examples There are four main types of catalysts: A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. An example. Catalyst Basic Examples.
From hxebeytdy.blob.core.windows.net
Heterogeneous Catalyst Chemistry Examples at Barbara Ward blog Catalyst Basic Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. Enzymes are naturally occurring catalysts responsible for many. An example of heterogeneous catalysis is the use. Catalyst Basic Examples.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Basic Examples Inorganic catalysts and organic catalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. There are two main types of catalysts: Catalysts play an essential role in our. Catalyst Basic Examples.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalyst Basic Examples Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. There are two main types of catalysts: An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with. Catalyst Basic Examples.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Basic Examples Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This lesson will give you a. There are four main types. Catalyst Basic Examples.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. This lesson will give you a. There are two main types of catalysts: There are four main types of catalysts: Catalysts play an essential role in our modern industrial economy, in our stewardship of the. Catalyst Basic Examples.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. There are two main types of catalysts: This lesson will give you a. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up. Catalyst Basic Examples.
From www.nagwa.com
Lesson Catalysts Nagwa Catalyst Basic Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. Catalyst Basic Examples.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. There are two main types of catalysts: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Inorganic catalysts and organic catalysts. For example, a catalyst could cause a. Catalyst Basic Examples.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Basic Examples Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Inorganic catalysts and organic catalysts. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy. Catalyst Basic Examples.
From www.slideshare.net
Catalyst Catalyst Basic Examples A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide. Catalyst Basic Examples.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Basic Examples Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. This lesson will give you a. Inorganic catalysts and organic catalysts. There are two main types of catalysts:. Catalyst Basic Examples.
From inspiritvr.com
CBSE Class 12 Chemistry Chapter 5 Revision Notes Inspirit Catalyst Basic Examples A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. There are four main types of catalysts: Inorganic catalysts and. Catalyst Basic Examples.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Basic Examples There are two main types of catalysts: Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all. Catalyst Basic Examples.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Basic Examples Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Enzymes are naturally occurring catalysts responsible for many. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. There are four main types of. Catalyst Basic Examples.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 Catalyst Basic Examples Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. There are two main types of catalysts: There are four main types of catalysts: A catalyst is a substance that increases the. Catalyst Basic Examples.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Basic Examples Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. There are four main types of catalysts: This lesson will give you a. Inorganic catalysts and organic catalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, a catalyst could cause a reaction between. Catalyst Basic Examples.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Catalyst Basic Examples There are four main types of catalysts: There are two main types of catalysts: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Enzymes are naturally occurring catalysts. Catalyst Basic Examples.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 Catalyst Basic Examples Inorganic catalysts and organic catalysts. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in. Catalyst Basic Examples.
From www.youtube.com
01.10 Base Catalysis and Summary YouTube Catalyst Basic Examples There are four main types of catalysts: Inorganic catalysts and organic catalysts. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the. Catalyst Basic Examples.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, a catalyst could cause a reaction between reactants to. Catalyst Basic Examples.
From www.slideserve.com
PPT Catalysts in Organic Synthesis PowerPoint Presentation, free Catalyst Basic Examples This lesson will give you a. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. There are two main types of catalysts: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of. Catalyst Basic Examples.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Basic Examples Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. There are four main types of catalysts: This lesson will give you a. There are two main types of catalysts: A catalyst is. Catalyst Basic Examples.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. There are two main types of catalysts: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Inorganic catalysts and organic catalysts. Enzymes are naturally occurring catalysts responsible for. Catalyst Basic Examples.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Basic Examples For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts play an essential role in our. Catalyst Basic Examples.
From www.slideserve.com
PPT Topic 06 6.2a Collision theory 6.2B Maxwell Catalyst Basic Examples For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst. Inorganic catalysts and organic catalysts. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. There are two main types of. Catalyst Basic Examples.
From www.youtube.com
What is Catalysis Catalyst Examples of Catalyst Class 11 YouTube Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. Inorganic catalysts and organic catalysts. This lesson will give you a. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. Enzymes are naturally. Catalyst Basic Examples.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Basic Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Enzymes are naturally occurring catalysts responsible for many. This lesson will give you a. There are two main types. Catalyst Basic Examples.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Catalyst Basic Examples Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. There are four main types of catalysts: There are two main types of catalysts:. Catalyst Basic Examples.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Catalyst Basic Examples An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. This lesson will give you a. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. There are four. Catalyst Basic Examples.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2823635 Catalyst Basic Examples This lesson will give you a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. There are two main types of catalysts: For example, a catalyst could cause a reaction between reactants. Catalyst Basic Examples.
From 2012books.lardbucket.org
Catalysis Catalyst Basic Examples There are two main types of catalysts: Enzymes are naturally occurring catalysts responsible for many. This lesson will give you a. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to. Catalyst Basic Examples.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Basic Examples Inorganic catalysts and organic catalysts. This lesson will give you a. There are two main types of catalysts: Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Contact catalysts are materials with the capability of adsorbing molecules of gases or liquids onto their surfaces. There are four main types of catalysts: Enzymes are naturally occurring catalysts responsible for many. Catalyst,. Catalyst Basic Examples.