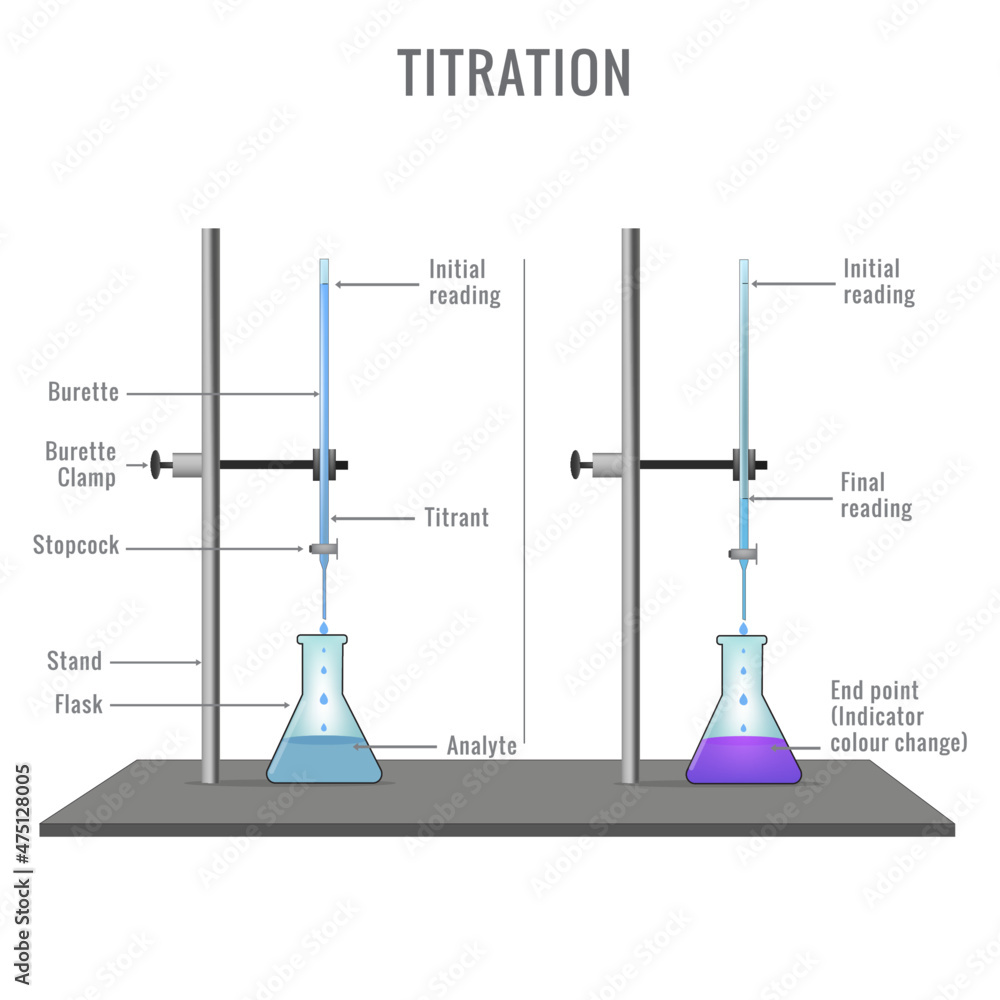
Titration Diagram To Label . Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. In an indicator based titration you add another. Figure is used with the permission of j.a. There are two basic types of acid base titrations, indicator and potentiometric. A ph curve is a graph showing how the ph of a solution changes as the acid (or. It is a quantitative technique based. During a titration a ph meter can be used and a ph curve plotted; In each case, you start with 25 cm 3 of one. The titration curve is a graph of the. Titrations involve the addition of the titrant from the burret to the analyte.
from stock.adobe.com
Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. In an indicator based titration you add another. In each case, you start with 25 cm 3 of one. It is a quantitative technique based. The titration curve is a graph of the. Figure is used with the permission of j.a. There are two basic types of acid base titrations, indicator and potentiometric. A ph curve is a graph showing how the ph of a solution changes as the acid (or. During a titration a ph meter can be used and a ph curve plotted; Titrations involve the addition of the titrant from the burret to the analyte.
Acid base titration experiment and phases of color change during
Titration Diagram To Label In an indicator based titration you add another. Figure is used with the permission of j.a. The titration curve is a graph of the. In each case, you start with 25 cm 3 of one. There are two basic types of acid base titrations, indicator and potentiometric. During a titration a ph meter can be used and a ph curve plotted; Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. Titrations involve the addition of the titrant from the burret to the analyte. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A ph curve is a graph showing how the ph of a solution changes as the acid (or. In an indicator based titration you add another. It is a quantitative technique based.
From mavink.com
Titration Diagram Labeled Titration Diagram To Label In each case, you start with 25 cm 3 of one. There are two basic types of acid base titrations, indicator and potentiometric. Titrations involve the addition of the titrant from the burret to the analyte. A ph curve is a graph showing how the ph of a solution changes as the acid (or. Determine the concentration of analyte present,. Titration Diagram To Label.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Diagram To Label There are two basic types of acid base titrations, indicator and potentiometric. During a titration a ph meter can be used and a ph curve plotted; The titration curve is a graph of the. Titrations involve the addition of the titrant from the burret to the analyte. In an indicator based titration you add another. It is a quantitative technique. Titration Diagram To Label.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Diagram To Label Titrations involve the addition of the titrant from the burret to the analyte. During a titration a ph meter can be used and a ph curve plotted; There are two basic types of acid base titrations, indicator and potentiometric. A ph curve is a graph showing how the ph of a solution changes as the acid (or. The titration curve. Titration Diagram To Label.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Diagram To Label The titration curve is a graph of the. A ph curve is a graph showing how the ph of a solution changes as the acid (or. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. It is a quantitative technique based. Determine the concentration of analyte. Titration Diagram To Label.
From www.chegg.com
Solved Label the titration diagram with the appropriate Titration Diagram To Label A ph curve is a graph showing how the ph of a solution changes as the acid (or. Figure is used with the permission of j.a. During a titration a ph meter can be used and a ph curve plotted; There are two basic types of acid base titrations, indicator and potentiometric. Titration is the process used to determine the. Titration Diagram To Label.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Diagram To Label The titration curve is a graph of the. A ph curve is a graph showing how the ph of a solution changes as the acid (or. In each case, you start with 25 cm 3 of one. It is a quantitative technique based. There are two basic types of acid base titrations, indicator and potentiometric. Determine the concentration of analyte. Titration Diagram To Label.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram To Label Titrations involve the addition of the titrant from the burret to the analyte. In an indicator based titration you add another. In each case, you start with 25 cm 3 of one. It is a quantitative technique based. There are two basic types of acid base titrations, indicator and potentiometric. The titration curve is a graph of the. During a. Titration Diagram To Label.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Diagram To Label It is a quantitative technique based. In an indicator based titration you add another. Titrations involve the addition of the titrant from the burret to the analyte. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. The titration curve is a graph of the. There are. Titration Diagram To Label.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Diagram To Label The titration curve is a graph of the. During a titration a ph meter can be used and a ph curve plotted; Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A ph curve is a graph showing how the ph of a solution changes as the acid (or. In each case, you. Titration Diagram To Label.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Diagram To Label It is a quantitative technique based. In an indicator based titration you add another. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. A ph curve is a graph showing how the ph of a solution changes as the acid (or. During a titration a ph. Titration Diagram To Label.
From edu.rsc.org
Vintage titrations sulfur dioxide concentrations in wine Resource Titration Diagram To Label During a titration a ph meter can be used and a ph curve plotted; Titrations involve the addition of the titrant from the burret to the analyte. It is a quantitative technique based. A ph curve is a graph showing how the ph of a solution changes as the acid (or. In each case, you start with 25 cm 3. Titration Diagram To Label.
From mavink.com
Titration Diagram Labeled Titration Diagram To Label It is a quantitative technique based. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. There are two basic types of acid base titrations, indicator and potentiometric. Figure is used with the permission of j.a. Titrations involve the addition of the titrant from the burret to the analyte. During a titration a ph. Titration Diagram To Label.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Diagram To Label Figure is used with the permission of j.a. During a titration a ph meter can be used and a ph curve plotted; A ph curve is a graph showing how the ph of a solution changes as the acid (or. It is a quantitative technique based. There are two basic types of acid base titrations, indicator and potentiometric. In an. Titration Diagram To Label.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram To Label There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. It is a quantitative technique based. A ph curve is a graph showing how the ph of a solution changes as the acid (or. Figure is used with the permission of j.a. Titrations involve the addition of the titrant from. Titration Diagram To Label.
From www.shutterstock.com
Titration Setup Educational Chemistry Diagram Stock Illustration Titration Diagram To Label Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. In each case, you start with 25 cm 3 of one. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. In an indicator based titration you add another. During a. Titration Diagram To Label.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Diagram To Label In an indicator based titration you add another. The titration curve is a graph of the. A ph curve is a graph showing how the ph of a solution changes as the acid (or. It is a quantitative technique based. Titrations involve the addition of the titrant from the burret to the analyte. There are two basic types of acid. Titration Diagram To Label.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Diagram To Label During a titration a ph meter can be used and a ph curve plotted; It is a quantitative technique based. Titrations involve the addition of the titrant from the burret to the analyte. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The titration curve is a graph of the. In each case,. Titration Diagram To Label.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Diagram To Label In an indicator based titration you add another. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A ph curve is a graph showing how the ph of a solution. Titration Diagram To Label.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram To Label The titration curve is a graph of the. Figure is used with the permission of j.a. During a titration a ph meter can be used and a ph curve plotted; Titrations involve the addition of the titrant from the burret to the analyte. It is a quantitative technique based. A ph curve is a graph showing how the ph of. Titration Diagram To Label.
From bramblechemistry.weebly.com
4C6 Titration Titration Diagram To Label Figure is used with the permission of j.a. A ph curve is a graph showing how the ph of a solution changes as the acid (or. During a titration a ph meter can be used and a ph curve plotted; In an indicator based titration you add another. It is a quantitative technique based. In each case, you start with. Titration Diagram To Label.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Diagram To Label In each case, you start with 25 cm 3 of one. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Titrations involve the addition of the titrant from the burret to the analyte. In an indicator based titration you add another. A ph curve is a graph showing how the ph of a. Titration Diagram To Label.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Diagram To Label Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. During a titration a ph meter can be used and a ph curve plotted; In an indicator based titration you add another. Figure is used with the permission of j.a. Titrations involve the addition of the titrant from the burret to the analyte. There. Titration Diagram To Label.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram To Label In an indicator based titration you add another. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. The titration curve is a graph of the. It is a quantitative technique based. There are two basic types of acid base titrations, indicator and potentiometric. In each case,. Titration Diagram To Label.
From stock.adobe.com
Acid base titration experiment and phases of color change during Titration Diagram To Label In each case, you start with 25 cm 3 of one. The titration curve is a graph of the. There are two basic types of acid base titrations, indicator and potentiometric. It is a quantitative technique based. During a titration a ph meter can be used and a ph curve plotted; In an indicator based titration you add another. Titrations. Titration Diagram To Label.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram To Label It is a quantitative technique based. In an indicator based titration you add another. A ph curve is a graph showing how the ph of a solution changes as the acid (or. The titration curve is a graph of the. In each case, you start with 25 cm 3 of one. There are two basic types of acid base titrations,. Titration Diagram To Label.
From www.youtube.com
Titration tube How to draw well labelled Titration Tube Titration Titration Diagram To Label In each case, you start with 25 cm 3 of one. Figure is used with the permission of j.a. During a titration a ph meter can be used and a ph curve plotted; In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Titration is the process used to determine. Titration Diagram To Label.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Diagram To Label During a titration a ph meter can be used and a ph curve plotted; In an indicator based titration you add another. A ph curve is a graph showing how the ph of a solution changes as the acid (or. It is a quantitative technique based. There are two basic types of acid base titrations, indicator and potentiometric. Determine the. Titration Diagram To Label.
From baharlabware.com
تیتراسیون و انواع آن تیتراسیون چیست محلول تیتراسیون بهار تجهیز Titration Diagram To Label The titration curve is a graph of the. In an indicator based titration you add another. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Figure is used with the permission of j.a. There are two basic types of acid base titrations, indicator and potentiometric. Titrations involve the addition of the titrant from. Titration Diagram To Label.
From www.savemyexams.co.uk
AcidBase Titrations (12.1.3) CIE IGCSE Chemistry Revision Notes 2023 Titration Diagram To Label It is a quantitative technique based. The titration curve is a graph of the. During a titration a ph meter can be used and a ph curve plotted; Titrations involve the addition of the titrant from the burret to the analyte. Titration is the process used to determine the volume of one solution that will react with a carefully measured. Titration Diagram To Label.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Diagram To Label Figure is used with the permission of j.a. There are two basic types of acid base titrations, indicator and potentiometric. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. The titration curve is a graph of the. Determine the concentration of analyte present, as well as. Titration Diagram To Label.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Diagram To Label A ph curve is a graph showing how the ph of a solution changes as the acid (or. During a titration a ph meter can be used and a ph curve plotted; Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution. In an indicator based titration. Titration Diagram To Label.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Diagram To Label Figure is used with the permission of j.a. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Titrations involve the addition of the titrant from the burret to the analyte. The titration curve is a graph of the. It is a quantitative technique based. There are two basic types of acid base titrations,. Titration Diagram To Label.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Diagram To Label A ph curve is a graph showing how the ph of a solution changes as the acid (or. In each case, you start with 25 cm 3 of one. Figure is used with the permission of j.a. Titration is the process used to determine the volume of one solution that will react with a carefully measured volume of another solution.. Titration Diagram To Label.
From mavink.com
Titration Apparatus Diagram Titration Diagram To Label Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. In each case, you start with 25 cm 3 of one. Titrations involve the addition of the titrant from the burret to the analyte. In an indicator based titration you add another. The titration curve is a graph of the. During a titration a. Titration Diagram To Label.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titration Diagram To Label There are two basic types of acid base titrations, indicator and potentiometric. Titrations involve the addition of the titrant from the burret to the analyte. During a titration a ph meter can be used and a ph curve plotted; It is a quantitative technique based. The titration curve is a graph of the. A ph curve is a graph showing. Titration Diagram To Label.