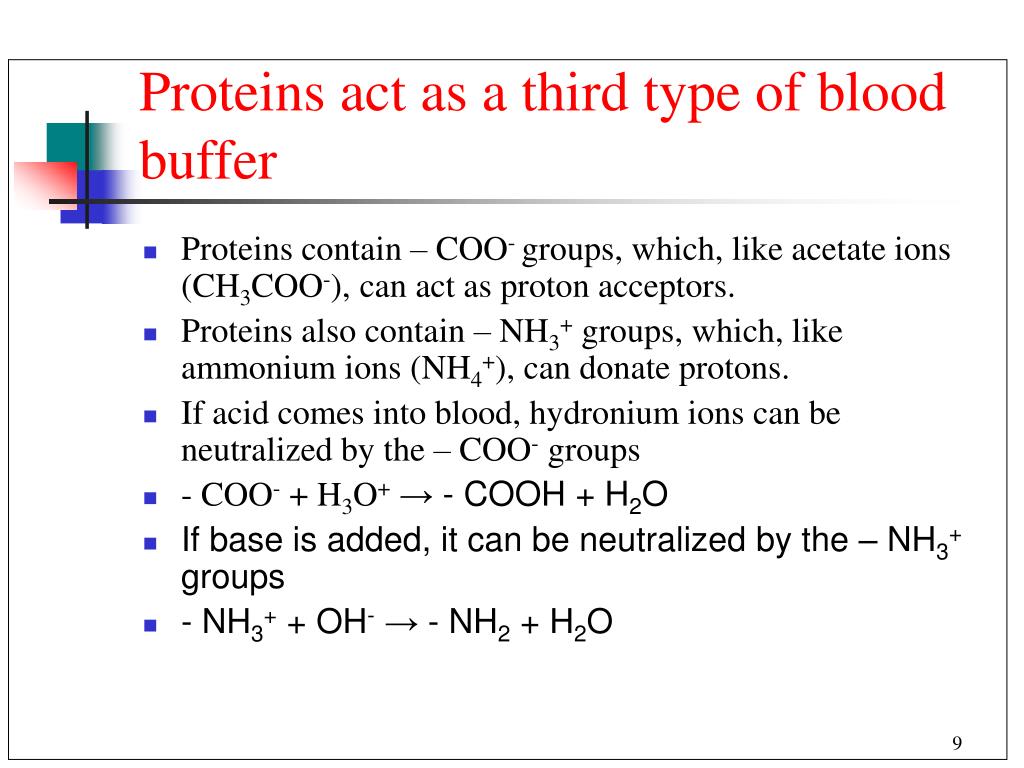
Which Buffer Is Present In Human Blood . One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood has a buffering system to minimize extreme changes in ph. Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +.
from www.slideserve.com
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. One buffer in blood is based on the presence of hco. Human blood has a buffering system to minimize extreme changes in ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffer systems work by neutralising added acid or base to resist changes to ph.
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation
Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffer systems work by neutralising added acid or base to resist changes to ph. Human blood has a buffering system to minimize extreme changes in ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One buffer in blood is based on the presence of hco.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID6910668 Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The. Which Buffer Is Present In Human Blood.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. Which Buffer Is Present In Human Blood.
From dokumen.tips
(PPT) Blood Buffers Module H Malley pages 120126. Objectives Define a Which Buffer Is Present In Human Blood Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID2412910 Which Buffer Is Present In Human Blood Human blood has a buffering system to minimize extreme changes in ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Buffer systems work by neutralising added acid or base to resist changes to ph. For example, when h + is. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One buffer in blood is based on the presence of hco. Buffer systems work by neutralising added acid or base to resist changes to ph. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Human. Which Buffer Is Present In Human Blood.
From pressbooks.bccampus.ca
26.4 AcidBase Balance Douglas College Human Anatomy and Physiology Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One buffer in blood is based on the presence of hco. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Buffer systems work by. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffer systems work by neutralising added acid or base to resist changes to ph. One buffer in blood is based on the presence of hco. The. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Salts in Solution Buffers PowerPoint Presentation, free download Which Buffer Is Present In Human Blood When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic. Which Buffer Is Present In Human Blood.
From slideplayer.com
Biological Buffers. ppt download Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One buffer in blood is based on the presence of hco. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Buffer systems work by. Which Buffer Is Present In Human Blood.
From www.youtube.com
The principal buffer present in human blood is 11 EQUILIBRIUM Which Buffer Is Present In Human Blood Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Human blood has a buffering system to minimize extreme changes in ph. For example, when h + is. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT AcidBase Balance I. PowerPoint Presentation, free download ID Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Human blood has a buffering system to minimize extreme changes in ph. The buffer systems. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood has a buffering system to minimize extreme changes in ph. One buffer in blood is based on the presence of hco. Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Salts in Solution Buffers PowerPoint Presentation, free download Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Human blood has a buffering system to minimize extreme changes in ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The buffer systems. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Which Buffer Is Present In Human Blood When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. One buffer in blood is based on the presence of hco. The buffer systems functioning. Which Buffer Is Present In Human Blood.
From facts.net
20 Fascinating Facts About Blood Buffer Which Buffer Is Present In Human Blood Buffer systems work by neutralising added acid or base to resist changes to ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. One buffer in blood is based on the presence of hco. The. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Which Buffer Is Present In Human Blood One buffer in blood is based on the presence of hco. Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. The buffer systems. Which Buffer Is Present In Human Blood.
From slideplayer.com
Acids and bases U N I T 9 Chapter ppt download Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. One buffer in blood is based on the presence of hco. Buffer systems work by. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood has a buffering system to minimize extreme changes in ph. When h +. Which Buffer Is Present In Human Blood.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. One buffer in blood is based on the presence of hco. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffer systems work by neutralising added acid or base to resist changes to ph. When. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Which Buffer Is Present In Human Blood Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One buffer in blood is based on the presence of hco. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffer systems work. Which Buffer Is Present In Human Blood.
From slideplayer.com
Unit 8 Part II Acids, Bases, pH, and Buffers Some Basic Chemistry for Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffer systems work by neutralising added acid or base to resist changes to ph. Human blood has a buffering system to minimize extreme changes in ph.. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT AcidBase Reactions Ch. 15 PowerPoint Presentation, free download Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Biological buffering of blood PowerPoint Presentation, free Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood has a buffering system to minimize extreme changes in ph. Buffer systems work by neutralising added acid or base to resist changes to ph. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +.. Which Buffer Is Present In Human Blood.
From www.slideshare.net
Blood buffer system PPT Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the. Which Buffer Is Present In Human Blood.
From en.ppt-online.org
Blood biochemistry online presentation Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One buffer in blood is based on the presence of hco. Buffer systems work by neutralising added acid or base to resist changes to ph. Human blood has a buffering system to minimize extreme changes in ph. For example, when h + is. Which Buffer Is Present In Human Blood.
From www.slideshare.net
02 hydrolysis. buffers__colloids Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffer systems work by neutralising added acid or base to resist changes to ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Which Buffer Is Present In Human Blood When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. For example, when. Which Buffer Is Present In Human Blood.
From slideplayer.com
Acids Lesson 20 Subtle Items. ppt download Which Buffer Is Present In Human Blood When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Human blood has a buffering system to minimize extreme changes in ph. Buffer systems work by neutralising added acid or base to resist changes to ph. The buffer systems functioning in blood. Which Buffer Is Present In Human Blood.
From www.doubtnut.com
[Odia] The principal buffer present in the blood is Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Human blood has a buffering system to minimize extreme changes in ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Buffer systems work. Which Buffer Is Present In Human Blood.
From bryont.net
Human Red Blood Cell Lysis Buffer Recipe Bryont Blog Which Buffer Is Present In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Human. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Which Buffer Is Present In Human Blood Buffer systems work by neutralising added acid or base to resist changes to ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. For. Which Buffer Is Present In Human Blood.
From www.youtube.com
Buffer action in the blood YouTube Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation Which Buffer Is Present In Human Blood One buffer in blood is based on the presence of hco. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Human blood has a buffering system to minimize extreme changes in ph. Buffer systems work by neutralising added acid or base. Which Buffer Is Present In Human Blood.
From slideplayer.com
Acid/Base Titration Buffers ppt download Which Buffer Is Present In Human Blood For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic. Which Buffer Is Present In Human Blood.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Which Buffer Is Present In Human Blood One buffer in blood is based on the presence of hco. Human blood has a buffering system to minimize extreme changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffer systems work. Which Buffer Is Present In Human Blood.