Electronegativity Of Chlorine And Fluorine . Fluorine possesses the highest electronegativity of all elements, and there is a decrease in electronegativity within the family of the. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. It can also be used to predict if the resulting molecule will be polar. The pauling scale is the most. You can also use our tool as an. It is an extremely reactive element and a strong oxidising agent:
from www.numerade.com
119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is an extremely reactive element and a strong oxidising agent: Fluorine possesses the highest electronegativity of all elements, and there is a decrease in electronegativity within the family of the. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. It can also be used to predict if the resulting molecule will be polar. You can also use our tool as an. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The pauling scale is the most.
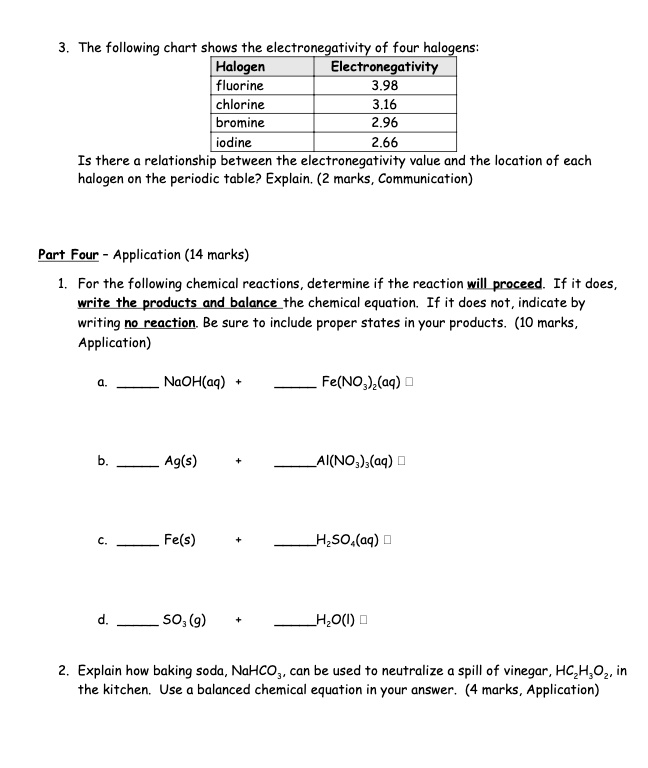
SOLVED The following chart shows the electronegativity of four halogens Halogens
Electronegativity Of Chlorine And Fluorine You can also use our tool as an. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. It can also be used to predict if the resulting molecule will be polar. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. You can also use our tool as an. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine possesses the highest electronegativity of all elements, and there is a decrease in electronegativity within the family of the. The pauling scale is the most. It is an extremely reactive element and a strong oxidising agent: As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID5631786 Electronegativity Of Chlorine And Fluorine The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. It is an extremely reactive element and a strong oxidising agent: Knowing that fluorine is the most. Electronegativity Of Chlorine And Fluorine.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Fluorine Electronegativity Of Chlorine And Fluorine 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus.. Electronegativity Of Chlorine And Fluorine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine is 3.6ev/ atom. The Electronegativity Of Chlorine And Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. The pauling scale is the most. You can also use our tool as an. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The electronegativity calculator allows you to. Electronegativity Of Chlorine And Fluorine.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Of Chlorine And Fluorine The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.. Electronegativity Of Chlorine And Fluorine.
From www.dreamstime.com
Fluorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Values Electronegativity Of Chlorine And Fluorine It is an extremely reactive element and a strong oxidising agent: Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. You can also use our tool as an. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Electronegativity. Electronegativity Of Chlorine And Fluorine.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Electronegativity Values Electronegativity Of Chlorine And Fluorine The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. It is an extremely reactive element and a strong oxidising agent: Knowing that fluorine is the most electronegative. Electronegativity Of Chlorine And Fluorine.
From socratic.org
Which group of elements is listed in order of increasing electronegativity? a) F, Cl, Ge, Sn b Electronegativity Of Chlorine And Fluorine The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron. Electronegativity Of Chlorine And Fluorine.
From www.numerade.com
SOLVED PLEASE HELP 15 POINTS AND MARKED BRAINLIST... Use the chart to determine which pair of Electronegativity Of Chlorine And Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. It can also. Electronegativity Of Chlorine And Fluorine.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Periodic Table Electronegativity Of Chlorine And Fluorine The pauling scale is the most. It is an extremely reactive element and a strong oxidising agent: Fluorine possesses the highest electronegativity of all elements, and there is a decrease in electronegativity within the family of the. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. As you go down a group,. Electronegativity Of Chlorine And Fluorine.
From maxim-has-huang.blogspot.com
Why Is Fluorine More Electronegative Than Chlorine MaximhasHuang Electronegativity Of Chlorine And Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. You can also use our tool as an. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4,. Electronegativity Of Chlorine And Fluorine.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Electronegativity Of Chlorine And Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Fluorine possesses the. Electronegativity Of Chlorine And Fluorine.
From www.numerade.com
SOLVED Define electronegativity and use Coulombs Law to compare the electronegativities of Electronegativity Of Chlorine And Fluorine 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. It is an extremely reactive element and a strong oxidising agent: You can also use our tool as an. The pauling scale is the most. Electronegativity. Electronegativity Of Chlorine And Fluorine.
From periodictable.me
How To Find Electron Configuration For Fluorine Dynamic Periodic Table of Elements and Chemistry Electronegativity Of Chlorine And Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. It is an extremely reactive element and a strong oxidising agent: The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. You can also use our tool. Electronegativity Of Chlorine And Fluorine.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Electronegativity Of Chlorine And Fluorine The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. It can also be used to predict if the resulting molecule will be polar. The pauling scale is the most. Fluorine. Electronegativity Of Chlorine And Fluorine.
From www.aiophotoz.com
Periodic Table Of Elements Electronegativity Chart Periodic Table Images and Photos finder Electronegativity Of Chlorine And Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. You can also use our tool as an. The pauling scale is the most. It can also be used to predict if the resulting molecule will be polar. Fluorine possesses the highest electronegativity of all elements, and there is a decrease in electronegativity. Electronegativity Of Chlorine And Fluorine.
From www.animalia-life.club
Electronegativity Energy Periodic Table Electronegativity Of Chlorine And Fluorine The pauling scale is the most. You can also use our tool as an. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is an extremely reactive element and a strong oxidising agent: Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. It. Electronegativity Of Chlorine And Fluorine.
From www.toppr.com
The electronegativity of fluorine obtained from the following data is x . EH H = 104.2 kcal Electronegativity Of Chlorine And Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The pauling scale is the most. You can also use our tool as an. It is an extremely reactive element. Electronegativity Of Chlorine And Fluorine.
From www.slideserve.com
PPT AS Chemistry PowerPoint Presentation ID2093824 Electronegativity Of Chlorine And Fluorine It is an extremely reactive element and a strong oxidising agent: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you to calculate the type of bond formed. Electronegativity Of Chlorine And Fluorine.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativityA. IncreasesB. First Electronegativity Of Chlorine And Fluorine It can also be used to predict if the resulting molecule will be polar. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Fluorine possesses the highest electronegativity of all elements, and there is a. Electronegativity Of Chlorine And Fluorine.
From www.ck12.org
Periodic Trends in Electronegativity CK12 Foundation Electronegativity Of Chlorine And Fluorine The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. It can also be used to predict if the resulting molecule will be polar. Fluorine possesses the highest electronegativity of all elements, and there is a decrease in electronegativity within the family of the. 119 rows electronegativity is used to. Electronegativity Of Chlorine And Fluorine.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Electronegativity Of Chlorine And Fluorine 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The pauling scale is the most. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron. Electronegativity Of Chlorine And Fluorine.
From www.youtube.com
Why electronegativity of chlorine is greater than of fluorine? Periodic TableNcert YouTube Electronegativity Of Chlorine And Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is an. Electronegativity Of Chlorine And Fluorine.
From askfilo.com
If the electronegativities of fluorine and chlorine are 4.0 and 3.0 resp.. Electronegativity Of Chlorine And Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. You can also use our tool as an. The electronegativity calculator allows you to calculate the type of bond formed between different elements. Electronegativity Of Chlorine And Fluorine.
From www.periodic-table.org
Chlorine Electronegativity Cl Electronegativity Of Chlorine And Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most. You. Electronegativity Of Chlorine And Fluorine.
From www.numerade.com
SOLVED The following chart shows the electronegativity of four halogens Halogens Electronegativity Of Chlorine And Fluorine The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. It is an extremely reactive element and a strong oxidising agent: Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The pauling scale is the most. Fluorine possesses the highest electronegativity of. Electronegativity Of Chlorine And Fluorine.
From sciencenotes.org
List of Electronegativity Values of the Elements Electronegativity Of Chlorine And Fluorine The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. You can also use our tool as an. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly. Electronegativity Of Chlorine And Fluorine.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Electronegativity Of Chlorine And Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The. Electronegativity Of Chlorine And Fluorine.
From byjus.com
why fluorine is more electronegative than oxygen. Electronegativity Of Chlorine And Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Fluorine possesses the. Electronegativity Of Chlorine And Fluorine.
From www.sliderbase.com
Pauling scale of electronegativity; Electronegativity Of Chlorine And Fluorine You can also use our tool as an. It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Knowing. Electronegativity Of Chlorine And Fluorine.
From mungfali.com
Electronegativity Chart And Lewis Structures Electronegativity Of Chlorine And Fluorine You can also use our tool as an. The pauling scale is the most. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. It is an extremely reactive element and a strong oxidising agent: As. Electronegativity Of Chlorine And Fluorine.
From slideplayer.com
Ionization Energy and Electron Affinity ppt download Electronegativity Of Chlorine And Fluorine 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. It can also be used to predict if the resulting molecule will be polar. Fluorine possesses the highest electronegativity of all elements, and there. Electronegativity Of Chlorine And Fluorine.
From www.britannica.com
Chemical compound Trends in the chemical properties of the elements Britannica Electronegativity Of Chlorine And Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is an. Electronegativity Of Chlorine And Fluorine.
From fluxdesignhouse.blogspot.com
How To Find Electronegativity Of An Element fluxdesignhouse Electronegativity Of Chlorine And Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The pauling scale is the most. It can also be used to predict if the resulting molecule will be polar. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to. Electronegativity Of Chlorine And Fluorine.
From www.gauthmath.com
Which element is most likely to react with chlorine? * ELECTRONEGATIVITY low medium high F [algebra] Electronegativity Of Chlorine And Fluorine You can also use our tool as an. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. It is an extremely reactive element and a strong oxidising agent: Electronegativity is a measure of. Electronegativity Of Chlorine And Fluorine.
From www.slideserve.com
PPT Why is Fluorine more electronegative than Carbon? PowerPoint Presentation ID7027942 Electronegativity Of Chlorine And Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity calculator allows you to. Electronegativity Of Chlorine And Fluorine.