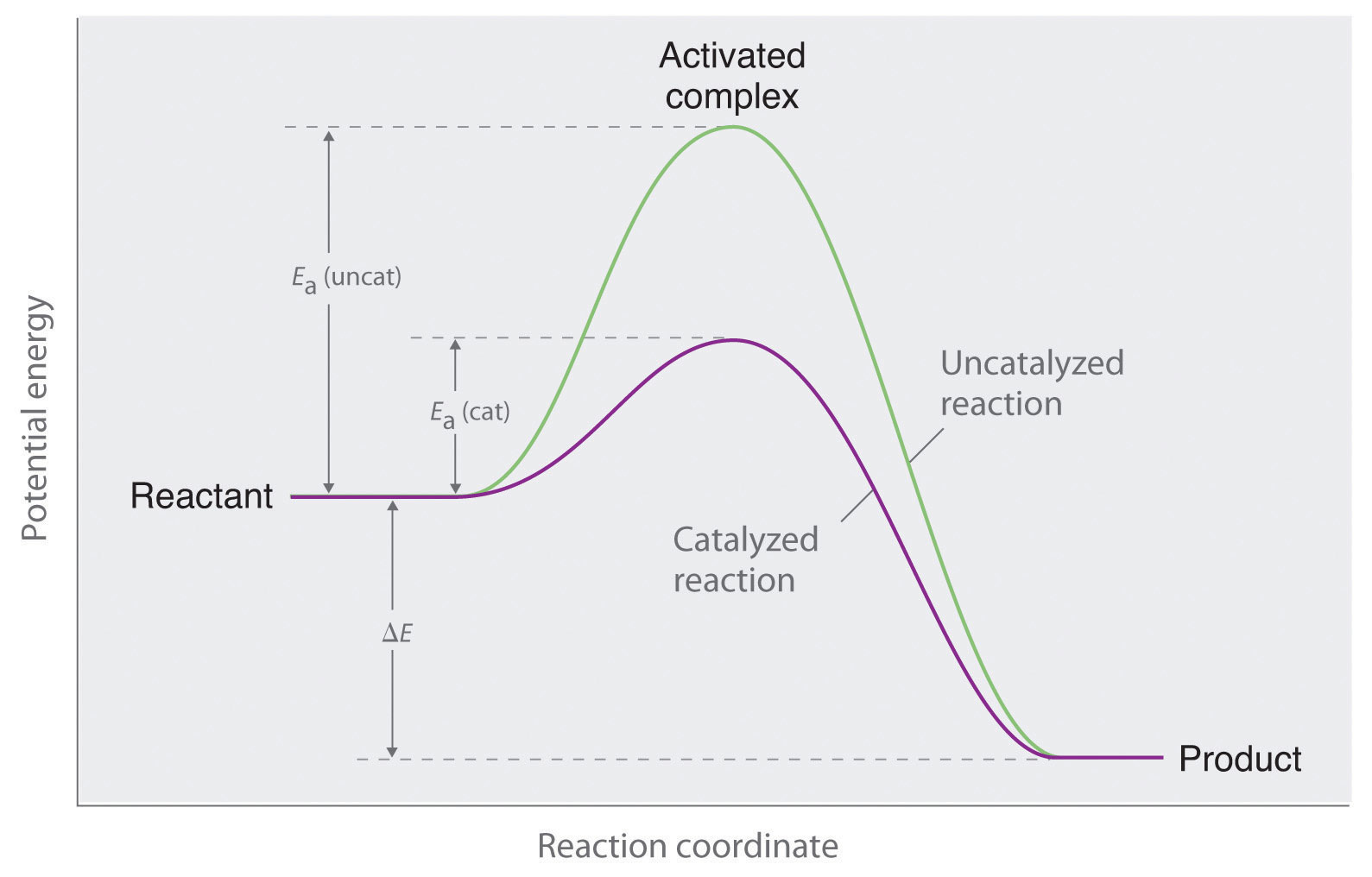
Does A Catalyst Increase The Rate Of The Reverse Reaction . In this section, we will examine the three major classes of catalysts: A catalyst is known to speed up both forward/backward reactions of a reversible reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. This page explains how adding a catalyst affects the rate of a reaction. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst also increases the rate of. But how does this work, because the mechanism. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants).
from 2012books.lardbucket.org
It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. This page explains how adding a catalyst affects the rate of a reaction. But how does this work, because the mechanism. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. In this section, we will examine the three major classes of catalysts: A catalyst also increases the rate of.
Catalysis
Does A Catalyst Increase The Rate Of The Reverse Reaction Heterogeneous catalysts, homogeneous catalysts, and enzymes. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. This page explains how adding a catalyst affects the rate of a reaction. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. A catalyst is known to speed up both forward/backward reactions of a reversible reaction. In this section, we will examine the three major classes of catalysts: This page describes and explains the way that adding a catalyst affects the rate of a reaction. But how does this work, because the mechanism. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. A catalyst also increases the rate of. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst is known to speed up both forward/backward reactions of a reversible reaction. But how does this work, because the mechanism. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. This page explains how adding. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. In this section, we will examine the three major classes of catalysts: Catalysts affect the rate of a. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Does A Catalyst Increase The Rate Of The Reverse Reaction But how does this work, because the mechanism. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). A catalyst also increases the rate of. With reference to the picture below, how is it. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From slideplayer.com
EQUILIBRIUM. ppt download Does A Catalyst Increase The Rate Of The Reverse Reaction Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. A catalyst also increases the rate of. But how does this work, because the mechanism. Catalysts affect the rate. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.numerade.com
SOLVEDIn general, how does a catalyst increase the rate of a chemical Does A Catalyst Increase The Rate Of The Reverse Reaction Heterogeneous catalysts, homogeneous catalysts, and enzymes. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). A catalyst also increases the rate of. But how does this work, because. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From loeljyzzv.blob.core.windows.net
Catalyst Chemical Reaction Rate at Leandro Jordan blog Does A Catalyst Increase The Rate Of The Reverse Reaction Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Catalysts. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst is known to speed up both forward/backward reactions of a reversible reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst also increases the rate of. This page describes and explains the way that adding a catalyst. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.savemyexams.com
The Position of Equilibrium Edexcel IGCSE Chemistry Revision Notes 2019 Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst is known to speed up both forward/backward reactions of a reversible reaction. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. But how does this work, because the mechanism. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Heterogeneous catalysts, homogeneous catalysts, and enzymes.. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.slideserve.com
PPT Chemical equilibrium occurs when a reaction and its reverse Does A Catalyst Increase The Rate Of The Reverse Reaction But how does this work, because the mechanism. In this section, we will examine the three major classes of catalysts: Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. This page explains how adding a catalyst affects the rate of a. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From byjus.com
How does a catalyst increase the rate of a reaction? Does A Catalyst Increase The Rate Of The Reverse Reaction This page explains how adding a catalyst affects the rate of a reaction. A catalyst also increases the rate of. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From gioyexizq.blob.core.windows.net
Does A Catalyst Increase The Rate Of A Reaction at Donald Gaffney blog Does A Catalyst Increase The Rate Of The Reverse Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. But how does this work, because the mechanism. A catalyst. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Does A Catalyst Increase The Rate Of The Reverse Reaction Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). With reference to the picture below,. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From exydadzyq.blob.core.windows.net
Catalyst Graph Chemistry at Margaret Wadlington blog Does A Catalyst Increase The Rate Of The Reverse Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Catalysts. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst also increases the rate of. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Heterogeneous catalysts,. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From 2012books.lardbucket.org
Catalysis Does A Catalyst Increase The Rate Of The Reverse Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). In this section, we. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. A catalyst is known to speed up both forward/backward reactions of a reversible. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst is known to speed up both forward/backward reactions of a reversible reaction. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. A catalyst also increases the rate of. It assumes that you are already. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.slideserve.com
PPT Chapter 12 Gaseous Chemical Equilibrium PowerPoint Presentation Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst is known to speed up both forward/backward reactions of a reversible reaction. Heterogeneous catalysts, homogeneous catalysts, and enzymes. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From loevndwvy.blob.core.windows.net
A Catalyst Increase The Rate Of A Reaction By at Carey Rains blog Does A Catalyst Increase The Rate Of The Reverse Reaction We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. In this section, we will examine the three major classes of catalysts: This page explains how adding a catalyst affects the rate of a reaction. Catalysts affect the rate of a chemical reaction by altering its mechanism to. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.youtube.com
Reverse Reaction Rate YouTube Does A Catalyst Increase The Rate Of The Reverse Reaction With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. A catalyst is known to speed up both forward/backward reactions of a reversible. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst also increases the rate of. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst is known to speed up both forward/backward reactions of a reversible reaction. With reference to the picture below, how is it that when. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Does A Catalyst Increase The Rate Of The Reverse Reaction Heterogeneous catalysts, homogeneous catalysts, and enzymes. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. This page explains how adding a catalyst affects the rate of a reaction. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Does A Catalyst Increase The Rate Of The Reverse Reaction Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. But how does this work, because the mechanism. This page explains how adding a catalyst affects the rate of a reaction. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst lowers the activation. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.chegg.com
Solved How does a catalyst increase the rate of a reaction? Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. In this section, we will examine the three major classes of catalysts: But how does this work, because the mechanism. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. With reference to the. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Does A Catalyst Increase The Rate Of The Reverse Reaction With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does A Catalyst Increase The Rate Of The Reverse Reaction In this section, we will examine the three major classes of catalysts: This page describes and explains the way that adding a catalyst affects the rate of a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. It assumes that. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Does A Catalyst Increase The Rate Of The Reverse Reaction We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed at a significant rate in response to even a. A catalyst also increases the rate of. A catalyst is known to speed up both forward/backward reactions of a reversible reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,.. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From gioyexizq.blob.core.windows.net
Does A Catalyst Increase The Rate Of A Reaction at Donald Gaffney blog Does A Catalyst Increase The Rate Of The Reverse Reaction Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. Because a catalyst decreases the height of. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From revisechemistry.uk
Reversible Reactions and Dynamic Equilibrium AQA C6 revisechemistry.uk Does A Catalyst Increase The Rate Of The Reverse Reaction This page explains how adding a catalyst affects the rate of a reaction. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. But how does this work, because the mechanism. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. We describe as ‘reversible’ a bidirectional catalyst. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Does A Catalyst Increase The Rate Of The Reverse Reaction This page explains how adding a catalyst affects the rate of a reaction. With reference to the picture below, how is it that when there is a lower activation energy (due to the alternate reaction pathway provided by the catalyst) that the catalyst will increase the. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does A Catalyst Increase The Rate Of The Reverse Reaction Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. But how does this work, because the mechanism. This page describes and explains the way that adding a. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From courses.lumenlearning.com
Catalysis Chemistry for Majors Does A Catalyst Increase The Rate Of The Reverse Reaction In this section, we will examine the three major classes of catalysts: It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. This page explains how adding a catalyst affects the rate of a reaction. Heterogeneous catalysts, homogeneous catalysts, and enzymes. With reference to the picture below, how is it that when there. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.slideshare.net
Rate of reaction(f5) Does A Catalyst Increase The Rate Of The Reverse Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst also increases the rate of. We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From www.gauthmath.com
Solved How does a catalyst increase the rate of a chemical reaction Does A Catalyst Increase The Rate Of The Reverse Reaction Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. In this section, we will examine the three major classes of catalysts: A catalyst also increases the rate of. A catalyst is known to speed up both forward/backward reactions of a reversible. Does A Catalyst Increase The Rate Of The Reverse Reaction.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Does A Catalyst Increase The Rate Of The Reverse Reaction A catalyst also increases the rate of. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. In this section, we will examine the three major classes of catalysts: We describe as ‘reversible’ a bidirectional catalyst that allows a reaction to proceed. Does A Catalyst Increase The Rate Of The Reverse Reaction.