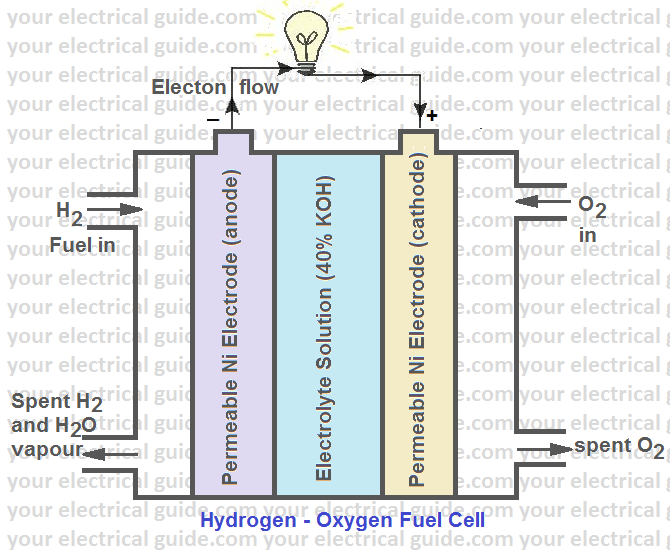
Hydrogen Fuel Cell Acidic Electrolyte . Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The fuel cell consists of. But the corrosion problem restricts the choice of construction materials. Based on the type of electrolyte used, fuel. Oxygen is supplied to a similar electrode except that the catalyst is. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global.
from www.yourelectricalguide.com
The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. The fuel cell consists of. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. Based on the type of electrolyte used, fuel. But the corrosion problem restricts the choice of construction materials.
Fuel Cell Working Principle your electrical guide
Hydrogen Fuel Cell Acidic Electrolyte The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. Oxygen is supplied to a similar electrode except that the catalyst is. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. The fuel cell consists of. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Based on the type of electrolyte used, fuel. But the corrosion problem restricts the choice of construction materials.
From wisc.pb.unizin.org
D42.5 Fuel Cells Chemistry 109 Fall 2021 Hydrogen Fuel Cell Acidic Electrolyte The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for. Hydrogen Fuel Cell Acidic Electrolyte.
From www.researchgate.net
Schematic of polymer electrolyte membrane (PEM) methanol electrolyzer Hydrogen Fuel Cell Acidic Electrolyte The fuel cell consists of. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Based on the type of electrolyte used, fuel. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Hydrogen enters the cell. Hydrogen Fuel Cell Acidic Electrolyte.
From www.slideserve.com
PPT BIOMASS FUELLED FUEL CELLS No Hydrogen Required Brant A. Peppley Hydrogen Fuel Cell Acidic Electrolyte Based on the type of electrolyte used, fuel. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e. Hydrogen Fuel Cell Acidic Electrolyte.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Hydrogen Fuel Cell Acidic Electrolyte Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. Based on the type of electrolyte used, fuel. Oxygen is supplied to a similar electrode except that the catalyst is. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e −. Hydrogen Fuel Cell Acidic Electrolyte.
From www.fuelcellstore.com
Fuel Cell Electrolyte Layer Modeling Hydrogen Fuel Cell Acidic Electrolyte Oxygen is supplied to a similar electrode except that the catalyst is. The fuel cell consists of. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. But the corrosion problem. Hydrogen Fuel Cell Acidic Electrolyte.
From large.stanford.edu
Hydrogen Fuel Cells Hydrogen Fuel Cell Acidic Electrolyte Oxygen is supplied to a similar electrode except that the catalyst is. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Based on the type of electrolyte used, fuel. The fuel cell consists of. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. But. Hydrogen Fuel Cell Acidic Electrolyte.
From www.chfca.ca
About Fuel Cells CHFCA Hydrogen Fuel Cell Acidic Electrolyte But the corrosion problem restricts the choice of construction materials. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is. The fuel cell. Hydrogen Fuel Cell Acidic Electrolyte.
From semiengineering.com
Fuel Cells And The IoE Hydrogen Fuel Cell Acidic Electrolyte Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. The fuel cell consists of. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. But the. Hydrogen Fuel Cell Acidic Electrolyte.
From www.slideserve.com
PPT Fuel Cells Fundamentals, Types, and Fuel Storage PowerPoint Hydrogen Fuel Cell Acidic Electrolyte But the corrosion problem restricts the choice of construction materials. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. Based on the type of electrolyte used, fuel. Oxygen is supplied to a similar electrode except that the catalyst is. The electrochemical reaction that occurs at the cathode is. Hydrogen Fuel Cell Acidic Electrolyte.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Hydrogen Fuel Cell Acidic Electrolyte The fuel cell consists of. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. But the corrosion problem restricts the choice of construction materials. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Based on the type. Hydrogen Fuel Cell Acidic Electrolyte.
From philschatz.com
Batteries and Fuel Cells · Chemistry Hydrogen Fuel Cell Acidic Electrolyte Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The fuel cell consists of. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global.. Hydrogen Fuel Cell Acidic Electrolyte.
From mavink.com
Proton Exchange Membrane Fuel Cell Diagram Hydrogen Fuel Cell Acidic Electrolyte The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Based on the type of electrolyte used, fuel. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Similar to batteries, a fuel cell is a device. Hydrogen Fuel Cell Acidic Electrolyte.
From crunchchemistry.co.uk
The chemistry of fuel cells Crunch Chemistry Hydrogen Fuel Cell Acidic Electrolyte Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. But the corrosion problem restricts the choice of construction materials. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Oxygen is supplied to a similar electrode except that. Hydrogen Fuel Cell Acidic Electrolyte.
From www.researchgate.net
Operation of alkaline fuel cell; the electrolyte used in this system is Hydrogen Fuel Cell Acidic Electrolyte The fuel cell consists of. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. The. Hydrogen Fuel Cell Acidic Electrolyte.
From chemistry.stackexchange.com
electrochemistry Hydrogen fuel cell why do the H+ ions move through Hydrogen Fuel Cell Acidic Electrolyte The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Based on the type of electrolyte used, fuel. Oxygen is supplied to a similar electrode except that. Hydrogen Fuel Cell Acidic Electrolyte.
From www.vrogue.co
Fuel Cell Basics Fuel Cell Hydrogen Energy Associatio vrogue.co Hydrogen Fuel Cell Acidic Electrolyte A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. But the corrosion problem restricts the choice of construction materials. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Hydrogen enters the cell through a. Hydrogen Fuel Cell Acidic Electrolyte.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Hydrogen Fuel Cell Acidic Electrolyte Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Similar to batteries, a fuel cell is a device that converts energy stored in. Hydrogen Fuel Cell Acidic Electrolyte.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Hydrogen Fuel Cell Acidic Electrolyte Based on the type of electrolyte used, fuel. But the corrosion problem restricts the choice of construction materials. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2. Hydrogen Fuel Cell Acidic Electrolyte.
From www.researchgate.net
The classical hydrogenoxygen fuel cell [5] Download Scientific Diagram Hydrogen Fuel Cell Acidic Electrolyte Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The fuel cell consists of. Based on the type of electrolyte used, fuel. Oxygen is supplied to a similar electrode except that the catalyst is. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical. Hydrogen Fuel Cell Acidic Electrolyte.
From www.yourelectricalguide.com
Fuel Cell Working Principle your electrical guide Hydrogen Fuel Cell Acidic Electrolyte Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Oxygen is supplied to a similar electrode except that the catalyst is. Similar to batteries, a fuel cell is a device that converts energy stored in. Hydrogen Fuel Cell Acidic Electrolyte.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Hydrogen Fuel Cell Acidic Electrolyte The fuel cell consists of. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. But the corrosion problem restricts the choice of construction materials. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Oxygen is supplied to a similar electrode except that the catalyst. Hydrogen Fuel Cell Acidic Electrolyte.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Hydrogen Fuel Cell Acidic Electrolyte But the corrosion problem restricts the choice of construction materials. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The fuel cell consists of. Oxygen is supplied to a similar electrode except that the. Hydrogen Fuel Cell Acidic Electrolyte.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Hydrogen Fuel Cell Acidic Electrolyte But the corrosion problem restricts the choice of construction materials. Oxygen is supplied to a similar electrode except that the catalyst is. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Based on the type of electrolyte used, fuel. Hydrogen enters the cell through a. Hydrogen Fuel Cell Acidic Electrolyte.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Hydrogen Fuel Cell Acidic Electrolyte But the corrosion problem restricts the choice of construction materials. Based on the type of electrolyte used, fuel. Oxygen is supplied to a similar electrode except that the catalyst is. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. The 2021 data reveals that europe. Hydrogen Fuel Cell Acidic Electrolyte.
From www.simscale.com
Hydrogen Fuel Cell Simulation & Modeling Blog SimScale Hydrogen Fuel Cell Acidic Electrolyte But the corrosion problem restricts the choice of construction materials. Based on the type of electrolyte used, fuel. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Similar to batteries, a fuel cell is. Hydrogen Fuel Cell Acidic Electrolyte.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Hydrogen Fuel Cell Acidic Electrolyte A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Based on the type of electrolyte used, fuel. The 2021 data reveals that europe is leading. Hydrogen Fuel Cell Acidic Electrolyte.
From web.stanford.edu
PEM Fuel Cells Hydrogen Fuel Cell Acidic Electrolyte The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. Hydrogen Fuel Cell Acidic Electrolyte.
From manualdiagramausterlitz.z19.web.core.windows.net
Fuel Cell Schematic Diagram Hydrogen Fuel Cell Acidic Electrolyte Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. Based on the type of electrolyte used, fuel. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 +. Hydrogen Fuel Cell Acidic Electrolyte.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Hydrogen Fuel Cell Acidic Electrolyte Oxygen is supplied to a similar electrode except that the catalyst is. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. But the corrosion problem restricts the choice of construction materials. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an. Hydrogen Fuel Cell Acidic Electrolyte.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Hydrogen Fuel Cell Acidic Electrolyte Based on the type of electrolyte used, fuel. But the corrosion problem restricts the choice of construction materials. The fuel cell consists of. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. Oxygen is supplied to a similar electrode except that the catalyst is. The electrochemical reaction that. Hydrogen Fuel Cell Acidic Electrolyte.
From chemistry.cornell.edu
Carboncoated nickel enables fuel cell free of precious metals Hydrogen Fuel Cell Acidic Electrolyte Oxygen is supplied to a similar electrode except that the catalyst is. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. But the corrosion problem restricts the choice of construction materials. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2. Hydrogen Fuel Cell Acidic Electrolyte.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne Hydrogen Fuel Cell Acidic Electrolyte The fuel cell consists of. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4 h 2 o. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell consists. Hydrogen Fuel Cell Acidic Electrolyte.
From www.energy.gov
Hydrogen Production Electrolysis Department of Energy Hydrogen Fuel Cell Acidic Electrolyte Based on the type of electrolyte used, fuel. Oxygen is supplied to a similar electrode except that the catalyst is. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. The electrochemical reaction that occurs at the cathode is 4 h + + o 2 + 4 e − → 4. Hydrogen Fuel Cell Acidic Electrolyte.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Hydrogen Fuel Cell Acidic Electrolyte The fuel cell consists of. Based on the type of electrolyte used, fuel. The 2021 data reveals that europe is leading the world in electrolyzer capacity deployment, accounting for 40% of global. Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. The electrochemical reaction that occurs at the. Hydrogen Fuel Cell Acidic Electrolyte.
From www.researchgate.net
Schematics of a) Alkaline Fuel Cell (AFC), b) Phosphoric Acid Fuel Cell Hydrogen Fuel Cell Acidic Electrolyte Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The fuel cell consists of. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an. Hydrogen Fuel Cell Acidic Electrolyte.