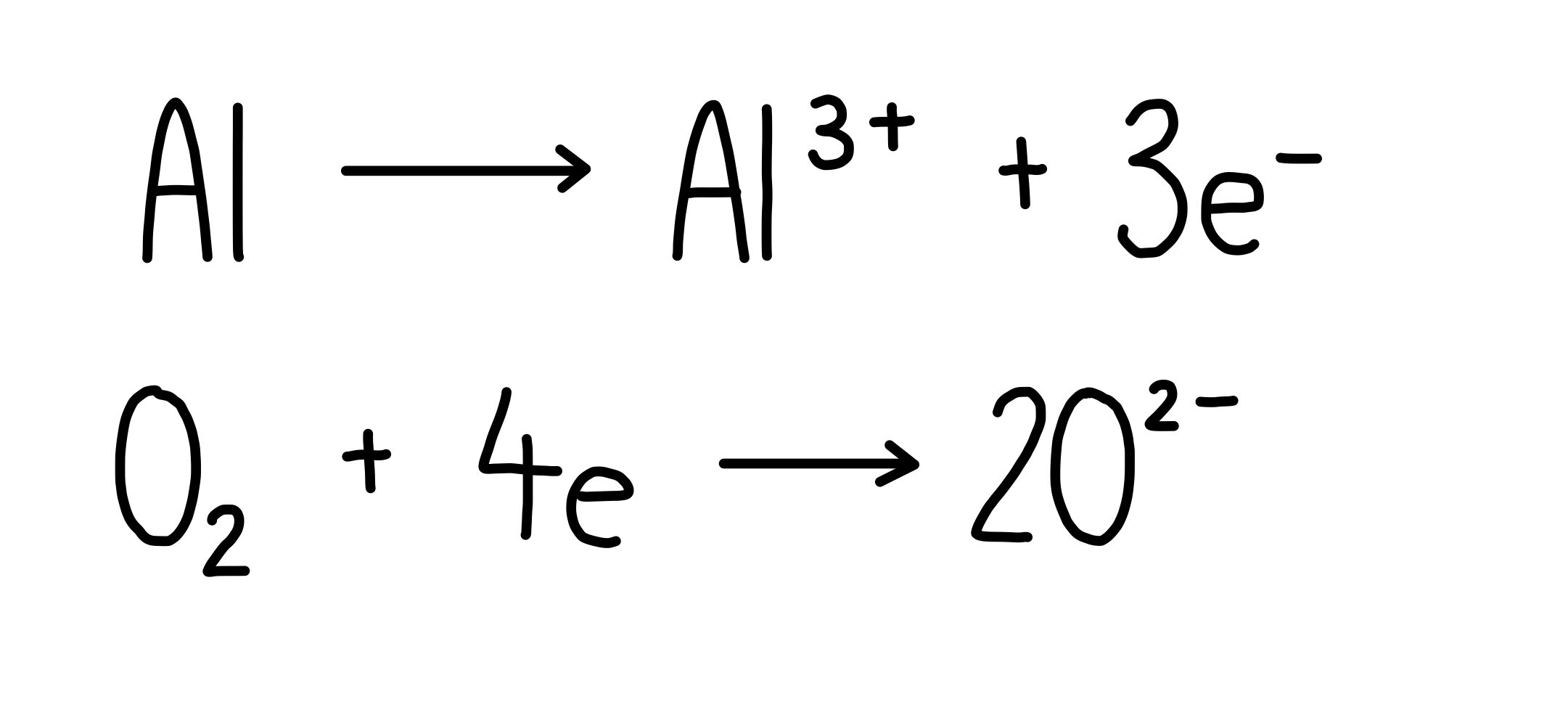Electrolysis Electrode Equations . Describe the process of electrolysis; Compare the operation of electrolytic cells with that of galvanic cells; An external voltage is applied to drive a nonspontaneous. Compare the operation of electrolytic cells with that of galvanic cells; The electrode attached to the positive. Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: To calculate the mass of a substance deposited at the electrode, you need to be able to: Unlike a voltaic cell, reactions using electrolytic cells must be. Describe the process of electrolysis; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged.
from www.thesciencehive.co.uk
Describe the process of electrolysis; The electrode attached to the positive. Describe the process of electrolysis; Compare the operation of electrolytic cells with that of galvanic cells; Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. Unlike a voltaic cell, reactions using electrolytic cells must be. Compare the operation of electrolytic cells with that of galvanic cells; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. In an electrolytic cell, however, the opposite process, called electrolysis, occurs:
Redox and Electrode Potentials — the science hive
Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; To calculate the mass of a substance deposited at the electrode, you need to be able to: Unlike a voltaic cell, reactions using electrolytic cells must be. At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. Describe the process of electrolysis; Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. Describe the process of electrolysis; In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a nonspontaneous. The electrode attached to the positive. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode.
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Electrolysis Electrode Equations The electrode attached to the positive. Compare the operation of electrolytic cells with that of galvanic cells; Describe the process of electrolysis; Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. To calculate the mass of a substance deposited at the electrode, you need to be able. Electrolysis Electrode Equations.
From www.meritnation.com
Predict the product of electrolysis in solution of H2SO4 with platinum Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. The electrode attached to the positive. Describe the process of electrolysis; An external voltage is applied to drive a nonspontaneous. Compare the operation of electrolytic cells with that of galvanic cells; The electrode. Electrolysis Electrode Equations.
From saylordotorg.github.io
Standard Potentials Electrolysis Electrode Equations The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; At the negative electrode (cathode), when the metal is more. Electrolysis Electrode Equations.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Electrolysis Electrode Equations In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Compare the operation of electrolytic cells with that of galvanic cells; Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. Describe the process of electrolysis; Describe the process of electrolysis; The electrode attached to the negative. Electrolysis Electrode Equations.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. Unlike a voltaic cell, reactions using electrolytic cells must be. Electrolytic cells consist of two electrodes. Electrolysis Electrode Equations.
From en.ppt-online.org
Electrolysis online presentation Electrolysis Electrode Equations An external voltage is applied to drive a nonspontaneous. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrode attached to the positive. Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells. Electrolysis Electrode Equations.
From www.nagwa.com
Question Video Determining the Equation That Shows the Reaction at the Electrolysis Electrode Equations The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; In an electrolytic cell, however, the opposite process, called electrolysis, occurs: To calculate the mass of a substance deposited at the. Electrolysis Electrode Equations.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Electrolysis Electrode Equations Describe the process of electrolysis; Compare the operation of electrolytic cells with that of galvanic cells; At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The electrode attached to the positive. To calculate the mass of. Electrolysis Electrode Equations.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrolysis Electrode Equations At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The electrode attached to the positive. Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic. Electrolysis Electrode Equations.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Electrode Equations An external voltage is applied to drive a nonspontaneous. The electrode attached to the positive. Describe the process of electrolysis; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Describe the process of electrolysis; Compare the operation of electrolytic cells with that of galvanic cells; Electrolytic cells consist of two electrodes (one. Electrolysis Electrode Equations.
From www.tessshebaylo.com
Electrolysis Of Salt Water Half Equations Tessshebaylo Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; Describe the process of electrolysis; Describe the process of electrolysis; Unlike a voltaic cell, reactions using electrolytic cells must be. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. To calculate. Electrolysis Electrode Equations.
From www.tessshebaylo.com
Electrolysis Of Brine Half Equations Tessshebaylo Electrolysis Electrode Equations To calculate the mass of a substance deposited at the electrode, you need to be able to: Compare the operation of electrolytic cells with that of galvanic cells; Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. In an electrolytic cell, however, the opposite process, called electrolysis,. Electrolysis Electrode Equations.
From www.youtube.com
Explaining the electrolysis of dilute sulfuric acid H2SO4 (aq) GCSE Electrolysis Electrode Equations At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a nonspontaneous. Compare the operation of electrolytic cells with that of galvanic cells; Describe the process of electrolysis; The electrode attached to the positive. Describe the. Electrolysis Electrode Equations.
From www.tessshebaylo.com
Electrolysis Of Salt Water Half Equations Tessshebaylo Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; Unlike a voltaic cell, reactions using electrolytic cells must be. Describe the process of electrolysis; An external voltage is applied to drive a nonspontaneous. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Describe the process of electrolysis; At the negative. Electrolysis Electrode Equations.
From www.simplechemconcepts.com
Simple Cells in Electrolysis topic Electrolysis Electrode Equations Describe the process of electrolysis; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Compare the operation of electrolytic cells with that of galvanic cells; At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. Describe the process of electrolysis; The electrode attached to the positive.. Electrolysis Electrode Equations.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrolysis Electrode Equations Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. Unlike a voltaic cell, reactions using electrolytic cells must be. Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; Describe the process of electrolysis; An. Electrolysis Electrode Equations.
From writeness-training.blogspot.com
Exam Questions On Electrolysis Electrolysis Electrode Equations The electrode attached to the positive. An external voltage is applied to drive a nonspontaneous. At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. Compare the operation of electrolytic cells with that of galvanic cells; Describe the process of electrolysis; To calculate the mass of a substance deposited at the electrode, you need. Electrolysis Electrode Equations.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets Electrolysis Electrode Equations In an electrolytic cell, however, the opposite process, called electrolysis, occurs: To calculate the mass of a substance deposited at the electrode, you need to be able to: Describe the process of electrolysis; Unlike a voltaic cell, reactions using electrolytic cells must be. Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as. Electrolysis Electrode Equations.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets Electrolysis Electrode Equations The electrode attached to the positive. To calculate the mass of a substance deposited at the electrode, you need to be able to: Describe the process of electrolysis; Compare the operation of electrolytic cells with that of galvanic cells; Unlike a voltaic cell, reactions using electrolytic cells must be. Describe the process of electrolysis; Electrolytic cells consist of two electrodes. Electrolysis Electrode Equations.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Electrode Equations Describe the process of electrolysis; Describe the process of electrolysis; An external voltage is applied to drive a nonspontaneous. To calculate the mass of a substance deposited at the electrode, you need to be able to: The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The electrode attached to the positive. Electrolytic. Electrolysis Electrode Equations.
From www.goodscience.com.au
Extraction of Metals Good Science Electrolysis Electrode Equations In an electrolytic cell, however, the opposite process, called electrolysis, occurs: To calculate the mass of a substance deposited at the electrode, you need to be able to: Describe the process of electrolysis; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An external voltage is applied to drive a nonspontaneous. At. Electrolysis Electrode Equations.
From hxectqqav.blob.core.windows.net
Electrolytic Electrode Charge at Keith Sullivan blog Electrolysis Electrode Equations To calculate the mass of a substance deposited at the electrode, you need to be able to: An external voltage is applied to drive a nonspontaneous. Compare the operation of electrolytic cells with that of galvanic cells; Unlike a voltaic cell, reactions using electrolytic cells must be. Electrolytic cells consist of two electrodes (one that acts as a cathode and. Electrolysis Electrode Equations.
From webmis.highland.cc.il.us
Electrolysis Electrolysis Electrode Equations At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; Describe the process of electrolysis; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode.. Electrolysis Electrode Equations.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Electrolysis Electrode Equations The electrode attached to the positive. Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. An external voltage is applied to drive a nonspontaneous. To calculate the mass of a substance deposited at the electrode, you need to be able to: The electrode attached to the negative. Electrolysis Electrode Equations.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrolysis Electrode Equations The electrode attached to the positive. Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. Describe the process of electrolysis; Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; The electrode attached to the. Electrolysis Electrode Equations.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; Compare the operation of electrolytic cells with that of galvanic cells; In an electrolytic cell, however, the opposite process, called electrolysis, occurs: To calculate the mass of a substance deposited at the electrode, you need to be able to: The electrode attached to the positive. An external voltage is. Electrolysis Electrode Equations.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrolysis Electrode Equations To calculate the mass of a substance deposited at the electrode, you need to be able to: The electrode attached to the positive. Compare the operation of electrolytic cells with that of galvanic cells; At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. An external voltage is applied to drive a nonspontaneous. The. Electrolysis Electrode Equations.
From byjus.com
Electrolysis of cuso4 solution using copper as electrode Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; The electrode attached to the positive. Describe the process of electrolysis; Describe the process of electrolysis; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts. Electrolysis Electrode Equations.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Electrolysis Electrode Equations The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. The electrode attached to the positive. Unlike a voltaic cell, reactions using electrolytic cells must be. Compare the operation of electrolytic cells with that of galvanic cells;. Electrolysis Electrode Equations.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube Electrolysis Electrode Equations The electrode attached to the positive. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Compare the operation of electrolytic cells with that of galvanic cells; To calculate the mass of a substance deposited at the electrode, you need to be able to: The electrode attached to the negative terminal of a battery is called a negative electrode,. Electrolysis Electrode Equations.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Electrolysis Electrode Equations At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. To calculate the mass of a substance deposited at the electrode, you need to be able to: Describe the process of electrolysis; The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The electrode attached to the. Electrolysis Electrode Equations.
From www.chemistrylearner.com
Electrolysis of Water Definition and Equation Electrolysis Electrode Equations To calculate the mass of a substance deposited at the electrode, you need to be able to: Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. Compare the operation of electrolytic cells with that of galvanic cells; Describe the process of electrolysis; Describe the process of electrolysis;. Electrolysis Electrode Equations.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrolysis Electrode Equations Compare the operation of electrolytic cells with that of galvanic cells; To calculate the mass of a substance deposited at the electrode, you need to be able to: An external voltage is applied to drive a nonspontaneous. Electrolytic cells consist of two electrodes (one that acts as a cathode and one that acts as an anode), and an electrolyte. In. Electrolysis Electrode Equations.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrolysis Electrode Equations At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. Unlike a voltaic cell, reactions using electrolytic cells must be. Compare the operation of electrolytic cells with that of galvanic cells; The electrode attached to the positive. Describe the process of electrolysis; Electrolytic cells consist of two electrodes (one that acts as a cathode. Electrolysis Electrode Equations.
From www.dreamstime.com
Electrolysis of Copper Sulfate Solution with Impure Copper Anode and Electrolysis Electrode Equations At the negative electrode (cathode), when the metal is more reactive than hydrogen, hydrogen is discharged. To calculate the mass of a substance deposited at the electrode, you need to be able to: Compare the operation of electrolytic cells with that of galvanic cells; An external voltage is applied to drive a nonspontaneous. Describe the process of electrolysis; In an. Electrolysis Electrode Equations.